Boiling point of dcm
Home » datasheet » Boiling point of dcmBoiling point of dcm
Boiling Point Of Dcm. 602-004-00-3 Component Classification Concentration Dichloromethane Skin Irrit. Unknown i re an dExpl os H z D t. It has been used for a wide range of samples like soils sediments and animal and plant tissues. 128 137 Drying the Organic Fraction pp.

So without a vigreux colomn he distilled the DCM and it came over at 39C until 41C. Soxhlet extraction is a simple and effective method. Unknown F Flash Point. It takes the form of straightforward advice in factsheets called control guidance sheets. It has been used for a wide range of samples like soils sediments and animal and plant tissues. Non-Flammable Lower Explosive Limit.
069 gmL Boiling point.
Solid caffeine can be then be isolated by evaporation of the low-boiling dichloromethane and identified by its melting point and mass spectrum. Appearance colorless liquid Density. 127 Solubility in Water. The mixture itself was 55C and the gastemperature was 39C and at the end 41C the mixture contained 65 DCM. The TVB bell has paddles inside and out. DCM is used as a solvent in the food industry and as a paint remover.
 Source: biopharma.co.uk
Source: biopharma.co.uk
Ethyl Ether 1 VOLATILES. The upper layer contains the less-dense solvent which in this case is toluene d 0867 gmL and the lower layer contains the denser solvent being water in this case. 100 Corrosion Rate IPY. The addition funnel is to get the DCM in the 1 liter round bottom flask. 1 2 0 Health 2.
 Source: future4200.com
Source: future4200.com
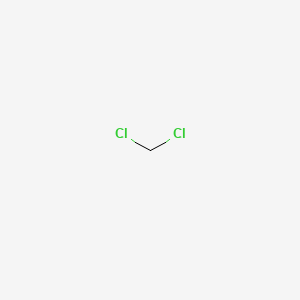
Boiling Temperature vs Chamber Pressure for Common Solvents-40-30-20-10 0 10 20 30 40 50 60 010 100 1000 10000. 60-100mg smoking 350mg orally Controlled substance hallucinogen US. Solid caffeine can be then be isolated by evaporation of the low-boiling dichloromethane and identified by its melting point and mass spectrum. 1 2 0 Health 2. DCM is used as a solvent in the food industry and as a paint remover.
98220 gmol 1. If you use DCM then it will have more DMF content so avoid using DCM. De Boer in Encyclopedia of Analytical Science Second Edition 2005 Soxhlet Extraction. -139F -95C VAPOUR PRESSURE. Dichloromethane is a member of the class of chloromethanes that is methane in which two of the hydrogens have been replaced by chlorineA dense non-flammible colourless liquid at room temperature bp.
 Source: researchgate.net
Source: researchgate.net
Winthrop University Organic Chemistry Lab Department of Chemistry CHEM 304 Revision 1-2015 Required Reading. 53 C 127 F. 137 140 Mass Spectrometry pp. NIOSH Pocket Guide International Chem Safety Card. The graph below shows a range of solvents with two well known HBP solvents DMF Dimethylformamide DMSO Dimethylsulfoxide occurring to the left of water which is in yellow on the graph.

Soxhlet extraction is a simple and effective method. Unknown F Flash Point. That should be good enough. You might expect to use approximately 10 -20 mL of water. 100 Corrosion Rate IPY.

STOT SE 3 H336. Soxhlet extraction is a simple and effective method. Boiling point of DCM. Unknown i re an dExpl os H z D t. - organic phase- DCM allyl bromide reactant 2-napthyl ether product benzyl tri-n-butylammonium chloride catalyst - inverted silica gel column TLC plate - Polar stays in the column non polar leaves product and allyl bromide Experiment 11.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Natural sources of dichloromethane include oceanic sources macroalgae wetlands and. Appearance colorless liquid Density. The liquid recondenses within the primary cooling circuit. There might be a little water in it so this time when you are boiling off the DCM let the temp of the distillation flask reach 50C for several minutes. For example if toluene is mixed with water a two-layer system is obtained.
 Source: acs.org
Source: acs.org
The servers in the demo featured Intel Habana Gaudi processors. STOT SE 3 H336. Set up for vacuum distillation and once again just boil off the DCM Boiling Stones. The liquid recondenses within the primary cooling circuit. De Boer in Encyclopedia of Analytical Science Second Edition 2005 Soxhlet Extraction.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Both the napthas have a boiling point greater than 100C the methanol 65C and the DCM 39-40C. A wide variety of solvents like dichloromethane DCM pure or mixed with acetone or hexane and acetonehexane mixtures can be used. The toluene layer along with its components. There are two types of sheets industry-specific direct advice sheets and generic control guidance. 100 Corrosion Rate IPY.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The liquid recondenses within the primary cooling circuit. The lower fluid boils and the bubbles rising through the other fluid create vibrational energy and dissipate the heat. Unknown i re an dExpl os H z D t. Recrystallize from boiling water in a 125 mL flask. As the compound is highly volatile in nature it can cause acute inhalation hazards.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of dcm by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.