Boiling point of cyclohexanol
Home » datasheet » Boiling point of cyclohexanolBoiling point of cyclohexanol
Boiling Point Of Cyclohexanol. Of 1390C but that of its isomer 2-methyl-2-butanol is 102oC. Billions of kilograms are produced annually mainly as a precursor to nylon. Pahlavan 3 The reaction is conducted in a distillation. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F.
 Cyclohexanol Structure Hazards Physical Properties Study Com From study.com
Cyclohexanol Structure Hazards Physical Properties Study Com From study.com
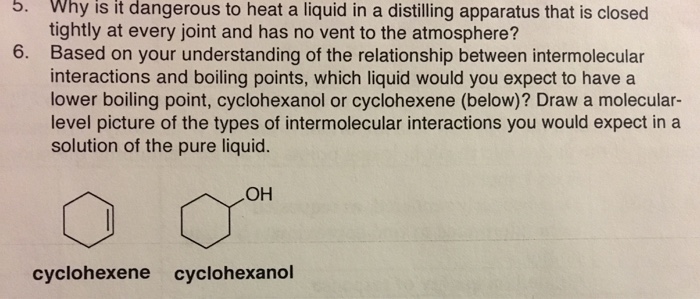
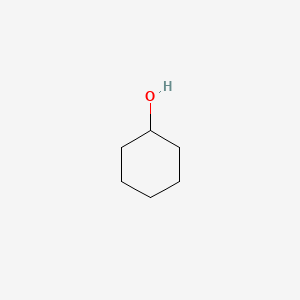
Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the. Chemistry of Natural Substances Organic Chemistry Worksheets 13 Worksheets 6 AlkanolsAlkyl halidesAlkanalsAlkanones and functional group tests Question 1. Cyclohexanol is the organic compound with the formula HOCHCH 2 5The molecule is related to cyclohexane by replacement of one hydrogen atom by a hydroxyl group. Determine the refractive index of the starting material cyclohexanol and your product cyclohexanone. Compare and contrast your FTIR versus the literature IR for the starting material and the product see below. Synthesis of Cyclohexene The Dehydration of Cyclohexanol.
Some products withstand extremely low temperatures of -100C and below.
H O - H. The oxidation involves radicals and the intermediacy of the hydroperoxide C 6 H 11 O 2 H. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220 bara. Make sure to. Van der Waals suggested a modification to take into account molecular size and molecular interaction forces.
 Source: chemsynthesis.com
Source: chemsynthesis.com
A Predict the products of the following reactions b Name the starting materials and. It becomes a vapor and is carried along by the helium carrier gas towards the detector. Temperature given as C F K and R. Chemistry of Natural Substances Organic Chemistry Worksheets 13 Worksheets 6 AlkanolsAlkyl halidesAlkanalsAlkanones and functional group tests Question 1. C MW 100 gmol MW 82 gmole d 096 gml d 081 gml step II step III step I.
Flash point 68 C 154 F - closed cup Ignition temperature 300 C 572 F Auto-ignition temperature no data available Lower explosion limit 125 V Upper explosion limit 1225 V. 20 - 22 C 68 - 72 F - lit. The distillate is collected at boiling temperature range of 77 C to 80C. A Predict the products of the following reactions b Name the starting materials and. Fractional distillation also ensures that the cyclohexene product boiling point 83C is not contaminated with the cyclohexanol starting material boiling point 161C.
 Source: study.com
Source: study.com
2 Construct a table of relevant information for reactants and products eg MPs BPs MWs densities hazardous properties. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. Temperature given as C F K and R. As it travels the second-lowest-boiling component may boil and begin traveling down the column as well behind the first fraction. In some cases purified cyclohexanol obtained by hydration of cyclohexene is the precursor.
Source: chemspider.com
2 Construct a table of relevant information for reactants and products eg MPs BPs MWs densities hazardous properties. Dehydration of Cyclohexanol Preparation of an Alkene CHM 220 PROCEDURE. The observed peaks for cyclohexanol were an O-H peak at 3400-3200 cm-1 and a C-H alkane peak at 3950-3850 cm-1. Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220 bara. Ethanol is soluble in water but cyclohexanol is not.
 Source: studylib.net
Source: studylib.net
The distillate is collected at boiling temperature range of 77 C to 80C. Temperature given as C F K and R. H O - H. Synthesis of Cyclohexene The Dehydration of Cyclohexanol. Even at temperatures at which organic rubbers turn brittle silicone rubber remains elastic.
 Source: researchgate.net
Source: researchgate.net
20 - 22 C 68 - 72 F - lit. Convert moles to grams and. The expected peaks for cyclohexanone were a CO peak between 1810-1640 cm-1 and a C-H alkane peak between 3000-2850 cm-1 1. Of 1390C but that of its isomer 2-methyl-2-butanol is 102oC. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly.
Source: pubchem.ncbi.nlm.nih.gov
1 Write out the balanced reaction using structural formulas. CHEM 2423 Cyclohexene Synthesis Dr. In some cases purified cyclohexanol obtained by hydration of cyclohexene is the precursor. This works well for dilute gases in many experimental circumstances. Chemistry of Natural Substances Organic Chemistry Worksheets 13 Worksheets 6 AlkanolsAlkyl halidesAlkanalsAlkanones and functional group tests Question 1.
1-pentanol has a bp. Because the cyclohexene has lower boiling point than the cyclohexanol the cyclohexene can be distilled as it forms. 20 - 22 C 68 - 72 F - lit. Compare and contrast your FTIR versus the literature IR for the starting material and the product see below. This electron pair delocalization is accompanied by a degree of rehybridization of the amino nitrogen atom but the electron pair delocalization is probably the major factor in the reduced basicity of these compounds.
 Source: en.wikipedia.org
Source: en.wikipedia.org
This works well for dilute gases in many experimental circumstances. Boiling Point ºC-886º. 160 - 161 deg. Cool the mixture to room temperature and add a few boiling chips to the cooled solution. In practice the distillate does not even boil at 83C because cyclohexene and water form an azeotrope whose boiling point is 70C.
Source:
The general approach towards carrying out an organic reaction. Synthesis of Cyclohexene The Dehydration of Cyclohexanol. In some cases purified cyclohexanol obtained by hydration of cyclohexene is the precursor. Convert moles to grams and. Fr om a 60 mL of cyclohexanol 229 grams of cyclo-hexene is produced.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of cyclohexanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.