Boiling point of ch4
Home » datasheet » Boiling point of ch4Boiling point of ch4
Boiling Point Of Ch4. Well BOTH molecules are highly symmetrical and thus have no resultant DIPOL. The main point of using the modnumpy functions is that they work element-wise on elements of an array. Of diamond 351 at 20C. Should you not have quoted the normal boiling points of the 2 solvents.
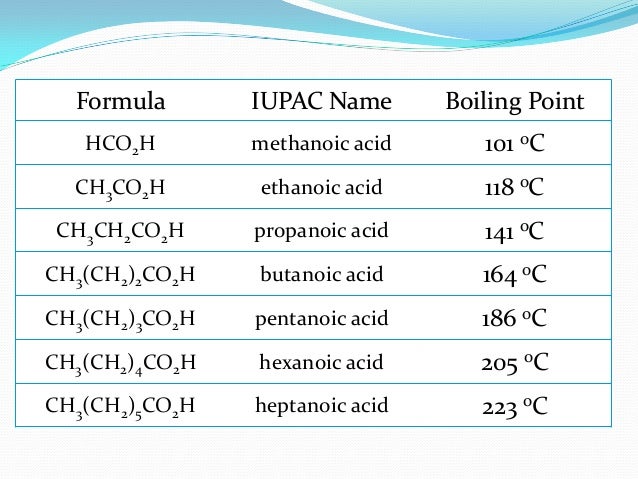 Determination Of Boiling Point Of Organic Compounds From pt.slideshare.net
Determination Of Boiling Point Of Organic Compounds From pt.slideshare.net
The webz says that the boiling point of methane is 16150 C. Of graphite 226 at 20C. It has the formula CF 3 CH 2 F and a boiling point of 263 C 1534 F at atmospheric pressure. B lower because the atmospheric pressure is lower. E The same because water always boils at 100 C Answer B. Only when hydrogen is loaded on a launch vehicle where no refrigeration exists it vents to the atmosphere.
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
Carbon definition a widely distributed element that forms organic compounds in combination with hydrogen oxygen etc and that occurs in a pure state as diamond and graphite and in an impure state as charcoal. A CH4. At higher altitudes the boiling point of water will be. D higher because there are fewer water molecules in the air. A CH3CH2CH2CH2OH B CH2CH2CH2OH C CH3CH2CH2CH3 D CH3CH2CH3 E CH3CH2CH2CH2CH2CH2CH2CH3. Now why the disparity.
 Source: youtube.com
Source: youtube.com
Arrange KCl NH3 and CH4 in order of increasing boiling point. A When 1 mole of CH4g reacts with O2g to form CO2g and H2Og according to. Covalent Bonds are in Liquid or gaseous State at room temperature. Should you not have quoted the normal boiling points of the 2 solvents. The stronger the dispersion forces the higher the boling point will be.
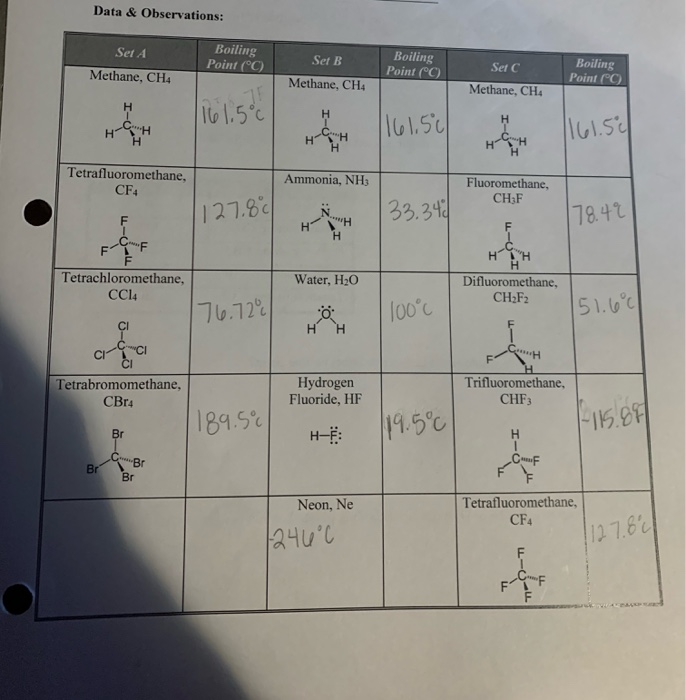 Source: chegg.com
Source: chegg.com
Also Check Difference Between Ionic Covalent and Metallic bonds. Distillation is a way to separate mixtures based upon differences in boiling point. 2 C Index of Refraction. Well BOTH molecules are highly symmetrical and thus have no resultant DIPOL. Natural Gas - Pipe Sizing - Sizing natural gas pipes - pressures above 5 psi 35 kPa.
 Source: quizlet.com
Source: quizlet.com
Explosions of such mixtures have been frequent in coal mines and collieries and have been the cause of many mine disasters. The energy content of 1 Nm hydrogen is equivalent to 034 l gasoline 1 l liquid hydrogen is equivalent to 027 l gasoline 1 kg hydrogen is equivalent to 275 kg gasoline based on lower heating value. 15 min for CH4 113 min for octane 147 min for the unknown and 190 min for nonane. A CH4 b KI c CS2 d HF e I2 6. Because of LNGs relatively high production cost.
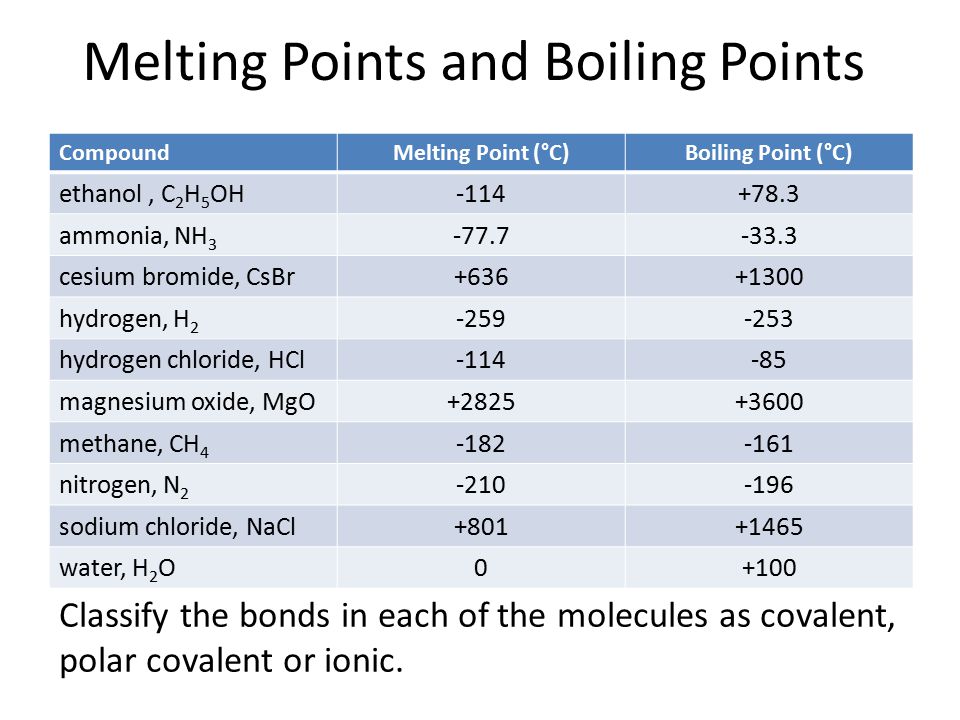 Source: slideplayer.com
Source: slideplayer.com
0 kJmol Flash Point. The main point of using the modnumpy functions is that they work element-wise on elements of an array. Drawing the Lewis structure for CH 4 named methane requires only single bondsIts one of the easier Lewis structures to draw. 0 kJmol Flash Point. Drawing the Lewis Structure for CH 4.

A When 1 mole of CH4g reacts with O2g to form CO2g and H2Og according to. Methane Gas - Specific Heat - Specific heat of Methane Gas - CH4 - at temperatures ranging 200 - 1100 K. In the late 1950s and early 1960s it was adopted for hydrogen-fuelled stages such as Centaur and Saturn upper stages. B lower because the atmospheric pressure is lower. Sodium chloride Sulfuric Acid.
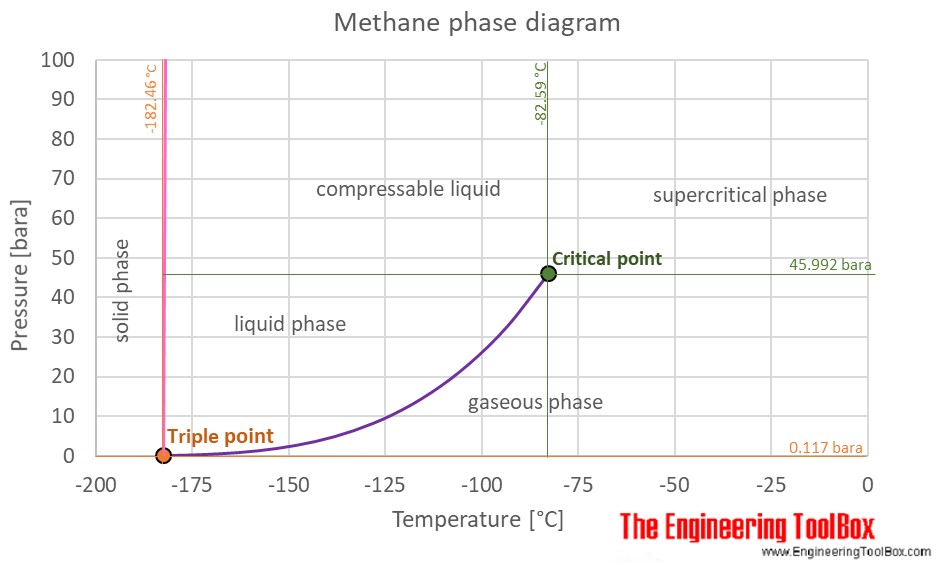 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Which compound is the most soluble in water. Nov 05 2009 CBr4 is larger than CH4 so it has a higher London dispersion a type of Intermolecular force. Calculate the energy required to heat 120mL of water for a cup of coffee to boiling point if the initial water temperatuer is 200C. Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220 bara. Drawing the Lewis structure for CH 4 named methane requires only single bondsIts one of the easier Lewis structures to draw.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Q m x c x ΔT Energy required to raise temperature of 120g of water by 1 degree SHC x mass 4184 x 120 5020J Since temperature rises by 80. 2 C Index of Refraction. The main point of using the modnumpy functions is that they work element-wise on elements of an array. Because of LNGs relatively high production cost. Of graphite 226 at 20C.
 Source: youtube.com
Source: youtube.com
What unit of. Now why the disparity. 0 kJmol Flash Point. Import numpy as np x nplinspace0 nppi 10 print npcosx You can already see from this output that there is a root to the equation cosx 0 because there is a change in sign in the output. In the late 1950s and early 1960s it was adopted for hydrogen-fuelled stages such as Centaur and Saturn upper stages.
 Source: reddit.com
Source: reddit.com
Which of the following pairs of compounds. Only when hydrogen is loaded on a launch vehicle where no refrigeration exists it vents to the atmosphere. Der Energieinhalt von 1 Nm Wasserstoff entspricht 034 l Benzin 1 l flüssiger Wasserstoff entspricht 027 l Benzin1 kg Wasserstoff entspricht 275 kg Benzin. Carbon definition a widely distributed element that forms organic compounds in combination with hydrogen oxygen etc and that occurs in a pure state as diamond and graphite and in an impure state as charcoal. Also Check Difference Between Ionic Covalent and Metallic bonds.
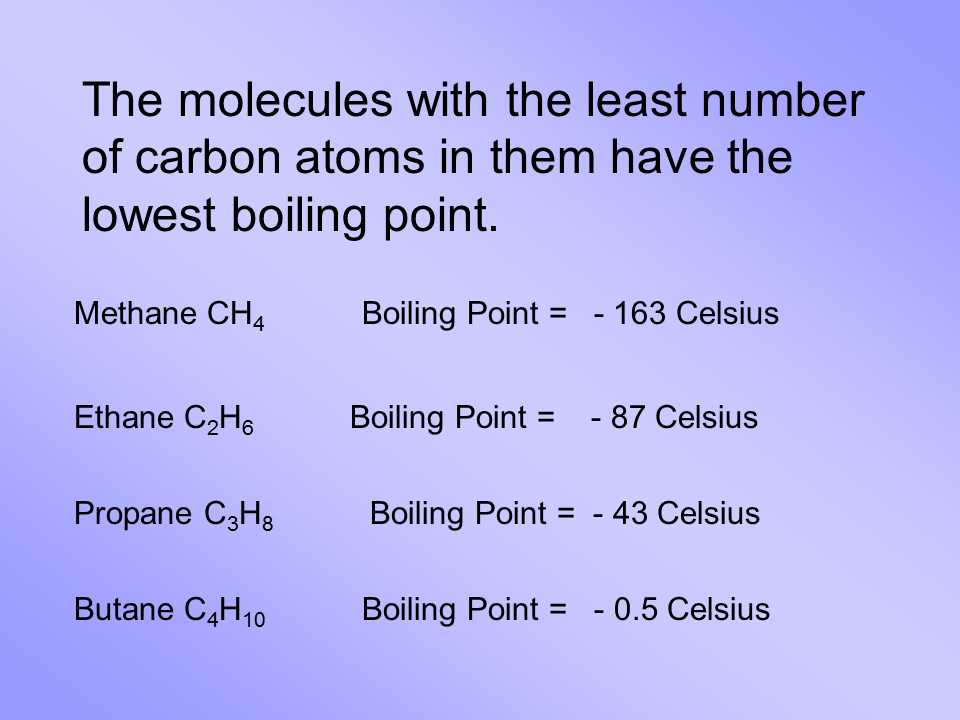
A CH4 b KI c CS2 d HF e I2 6. A CH4 b KI c CS2 d HF e I2 6. For CH 4 you have a total of 8 total valence electrons. Sodium chloride Sulfuric Acid. A lower because temperatures are lower.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of ch4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.