Boiling point of cf4
Home » datasheet » Boiling point of cf4Boiling point of cf4
Boiling Point Of Cf4. Total Valence electron for CF4. 2 C Index of Refraction. 25 11 17 19 and 19 compounds 22. Specific Heat Helmholtz Energy Gibbs Free Energy Joule-Thomson Coefficient Thermal Conductivity Surface Tension Standard Boiling Point Triple Point Critical Temperature Critical Pressure Acentric Factor Dipole Moment NIST Saturated Vapor Pressure Adiabatic Exponent.
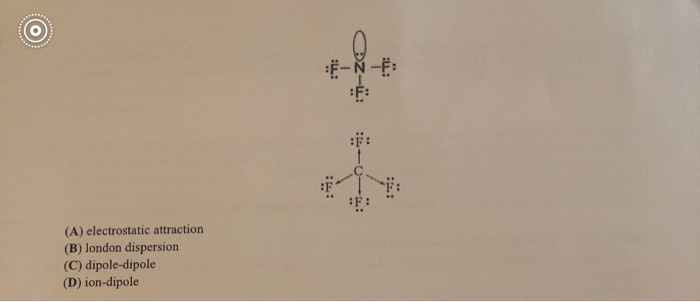 Solved 27 Nf3 Has A Higher Boiling Point Than Cf4 Based On Chegg Com From chegg.com
Solved 27 Nf3 Has A Higher Boiling Point Than Cf4 Based On Chegg Com From chegg.com
Assuming that the reaction occurs at constant pressure how much heat is released. Used to make other chemicals. It has a boiling point of 1897 C and a melting point of 945 C. This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. Low melting and boiling points. Much more dense than water and insoluble in water.
When determining between polar and nonpolar.
Specific Heat Helmholtz Energy Gibbs Free Energy Joule-Thomson Coefficient Thermal Conductivity Surface Tension Standard Boiling Point Triple Point Critical Temperature Critical Pressure Acentric Factor Dipole Moment NIST Saturated Vapor Pressure Adiabatic Exponent. Lewis structure for h2ccch2. And boil The End Chemical Bond A bond results from the attraction of nuclei for electrons All atoms trying to achieve a stable octet IN OTHER WORDS the p in one nucleus are attracted to the e. Nonpolar Total Valence electron for CBr4. CF4 to have the highest boiling point and the lowest vapor pressure CF4 to have the highest boiling point and the highest vapor pressure CF4 to have the lowest boiling point and the highest vapor pressure CF4 to have the lowest. CF4 R141B CCl2FCH3 R142B CClF2CH3 R143A CF3CH3 R152A CHF2CH3 R161 C2H5F R170 CH3CH3 R21 CHCl2F R218.
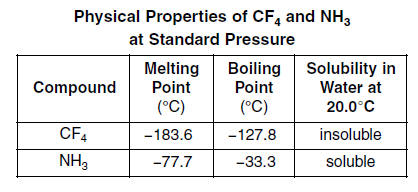 Source: kentchemistry.com
Source: kentchemistry.com
Electron geometry of CBr4. Consequently this method allows for reliable straight-forward quantitation of NF3CF4 mixtures which is necessary when studying the commercially important problem of NF3 and CF4 separation by different methods. The EJ251 and EJ252 engines had two ignition coils one for each pair of cylinders ie. Cuf2 is a meiotic transcription factor and its critical target is fzr1 mfr1 which encodes a meiotic APCC activator. Feb 10 2020 This is the basis for the difference between polar and nonpolar bonds.
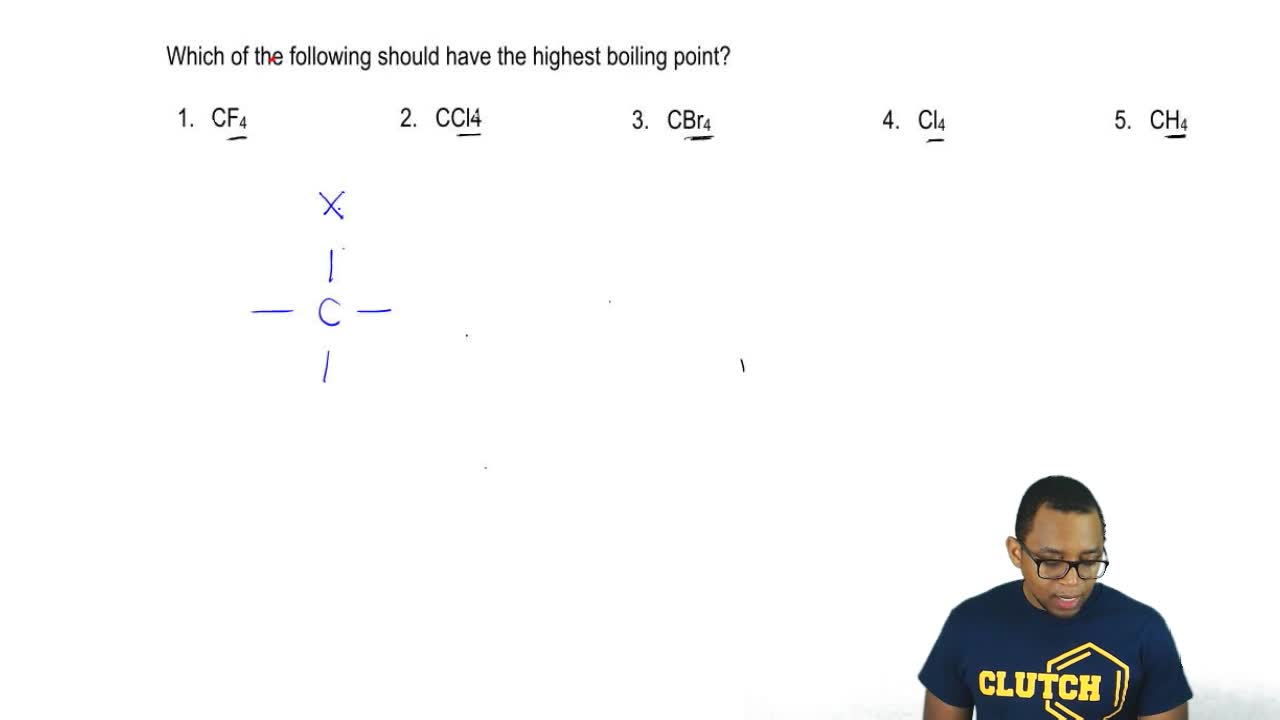 Source: clutchprep.com
Source: clutchprep.com
Consequently this method allows for reliable straight-forward quantitation of NF3CF4 mixtures which is necessary when studying the commercially important problem of NF3 and CF4 separation by different methods. That of carbon tetrachloride is 7672 C. Now why the disparity. Assuming that the reaction occurs at constant pressure how much heat is released. Fluorine has the highest e-neg SO HF will experience the strongest H bonding and needs the most energy to weaken the imf.

That of carbon tetrachloride is 7672 C. It is denser than water. 0 kJmol Flash Point. Free essays homework help flashcards research papers book reports term papers history science politics. Used to make other chemicals.
 Source: slideplayer.com
Source: slideplayer.com
A symmetry element is a line a plane or a point in or through an object about which a rotation or reflection leaves the object in an orientation indistinguishable from the original. Feb 16 2020 Likewise which has a higher boiling point CCl4 or CBr4. Enter the email address you signed up with and well email you a reset link. Solution We write the Lewis structure of NH 4 as. Low melting and boiling points.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. Now why the disparity. Feb 10 2020 This is the basis for the difference between polar and nonpolar bonds. 0 grams of methane CH4. Should you not have quoted the normal boiling points of the 2 solvents.
 Source: slideplayer.com
Source: slideplayer.com
Whether a molecule is polar or non-polar can make a difference in several ways. Nonpolar Total Valence electron for CBr4. How to draw lewis structure for CF4 Carbon. Feb 16 2020 Likewise which has a higher boiling point CCl4 or CBr4. CCl₄ will dissolve in Benzene The SEA water model computes the free energies of solvation of nonpolar and polar solutes in water with good efficiency and accuracy.
 Source: chegg.com
Source: chegg.com
Please help Suppose that 0250mol of methane CH4g is reacted with 0400mol of fluorine F2g forming CF4g and HFg as sole products. Consequently this method allows for reliable straight-forward quantitation of NF3CF4 mixtures which is necessary when studying the commercially important problem of NF3 and CF4 separation by different methods. Iodomethane CH3I is asymmetric and polar with a boiling point of 42 C. Assuming that the reaction occurs at constant pressure how much heat is released. 2 C Index of Refraction.
 Source: slideplayer.com
Source: slideplayer.com
Petroleum ether a petroleum distillation fraction is a mixture of low molecular weight aliphatic hydrocarbons mostly pentanes and hexanes with a low boiling range typically around 30-60oC. 0 kJmol Flash Point. CF4 R141B CCl2FCH3 R142B CClF2CH3 R143A CF3CH3 R152A CHF2CH3 R161 C2H5F R170 CH3CH3 R21 CHCl2F R218. You can view more similar questions or ask a new question. Fluorine has the highest e-neg SO HF will experience the strongest H bonding and needs the most energy to weaken the imf.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Of the compounds CF4 CCl4 CBr4 and CI4 we would expect. Our Range includes Brake Fluid Coolant Gear Oil Hydraulic Oil Metal Working Fluids Gasoline Diesel Engine Oil. Petroleum ether is commonly used as a nonpolar. Should you not have quoted the normal boiling points of the 2 solvents. Fluorine has the highest e-neg SO HF will experience the strongest H bonding and needs the most energy to weaken the imf.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Pembuatan Senyawa kompleks asetial asetanoat. The boiling point of H2O is drastically higher than the other similar compounds. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. Assuming that the reaction occurs at constant pressure how much heat is released. It has a monoclinic tetrahedral structure.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of cf4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.