Boiling point of carbon disulfide
Home » datasheet » Boiling point of carbon disulfideBoiling point of carbon disulfide
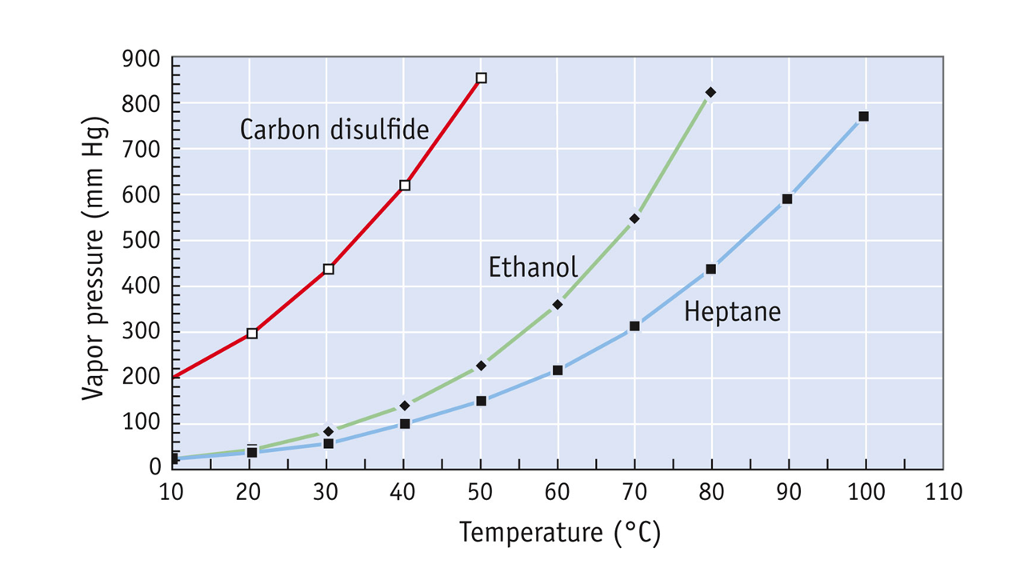
Boiling Point Of Carbon Disulfide. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid. For compounds of the same class the boiling temperature increases with carbon number.

For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. While examining adsorption of mercaptan on activated carbon Ishizaki and Cookson 1974 illustrated that activated carbon catalyzed the oxidation of mercaptan to disulfide. For compounds with the same carbon number the order of increasing boiling point by class is isoparaffin n-paraffin naphthene and aromatic. Boiling point - the temperature at which a liquid turns into a gas. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. The main target users are workers and those responsible for occupational safety and health.
Physical Thermo-dynamic Environmental.
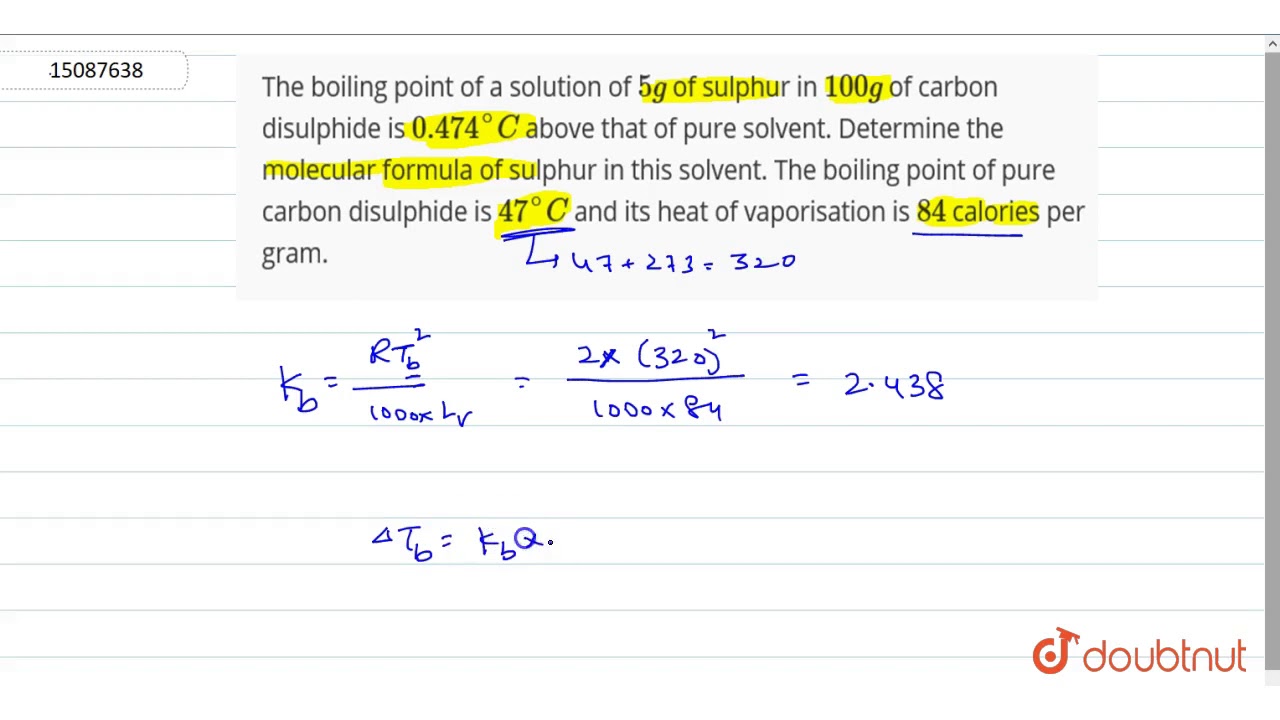
Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. They confirmed that the disulfide competes with mercaptan for sorption sites on activated carbon. Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. Here T BP is the boiling point elevation– the change in the boiling point that occurs when a solute dissolves in the solvent and k b is a proportionality constant known as the molal boiling point elevation constant for the solvent. Boiling and freezing temperatures of selected compounds of diesel fuel are listed in Table 2. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
 Source: clutchprep.com
Source: clutchprep.com
The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid. They confirmed that the disulfide competes with mercaptan for sorption sites on activated carbon. Adsorption equilibrium constants were established for both the mercaptan and disulfide. The main target users are workers and those responsible for occupational safety and health. In light of this information it.
 Source: clutchprep.com
Source: clutchprep.com
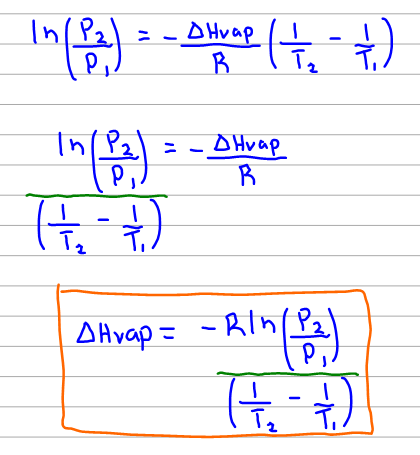
A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. For compounds with the same carbon number the order of increasing boiling point by class is isoparaffin n-paraffin naphthene and aromatic. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure.
 Source: oneclass.com
Source: oneclass.com
Adsorption equilibrium constants were established for both the mercaptan and disulfide. Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solventIt has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities. Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. The ICSC project is a common undertaking between the World Health Organization WHO and. Carbon combines with some metals at high temperatures to form metallic carbides such as the iron carbide cementite in steel and tungsten carbide widely used as an abrasive and for making hard tips for cutting tools.
 Source: study.com
Source: study.com
For compounds of the same class the boiling temperature increases with carbon number. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Here T BP is the boiling point elevation– the change in the boiling point that occurs when a solute dissolves in the solvent and k b is a proportionality constant known as the molal boiling point elevation constant for the solvent. Boiling point - the temperature at which a liquid turns into a gas. For compounds of the same class the boiling temperature increases with carbon number.
 Source: bartleby.com
Source: bartleby.com
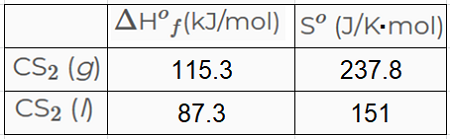
Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solventIt has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. Boiling and freezing temperatures of selected compounds of diesel fuel are listed in Table 2. The main target users are workers and those responsible for occupational safety and health. The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid.
 Source: chegg.com
Source: chegg.com
Melting point - the temperature at which a solid turns into a liquid. Carbon disulfide also spelled as carbon disulphide is a neurotoxic colorless volatile liquid with the formula CS 2The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solventIt has an ether-like odor but commercial samples are typically contaminated with foul-smelling impurities. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Carbon reacts with sulfur to form carbon disulfide and it reacts with steam in the coal-gas reaction used in coal gasification. They confirmed that the disulfide competes with mercaptan for sorption sites on activated carbon.
 Source: chegg.com
Source: chegg.com
A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. They confirmed that the disulfide competes with mercaptan for sorption sites on activated carbon. Boiling point - the temperature at which a liquid turns into a gas. Boiling and freezing temperatures of selected compounds of diesel fuel are listed in Table 2. The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. While examining adsorption of mercaptan on activated carbon Ishizaki and Cookson 1974 illustrated that activated carbon catalyzed the oxidation of mercaptan to disulfide. The primary aim of the cards is to promote the safe use of chemicals in the workplace. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Carbon combines with some metals at high temperatures to form metallic carbides such as the iron carbide cementite in steel and tungsten carbide widely used as an abrasive and for making hard tips for cutting tools.

Yaws CL Chemical Properties Handbook. Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. C s H 2 O g CO g H 2g. In light of this information it. T FP -k.
 Source: youtube.com
Source: youtube.com
Of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. The main target users are workers and those responsible for occupational safety and health. T FP -k. Melting point - the temperature at which a solid turns into a liquid. Yaws CL Chemical Properties Handbook.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of carbon disulfide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.