Boiling point of carbon
Home » datasheet » Boiling point of carbonBoiling point of carbon
Boiling Point Of Carbon. 1560 626 306 Camphor. It takes more energy to overcome the force of attraction and so the boiling point rises. The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid. Lead for example has such a low melting point that its easily liquefied by a flame.
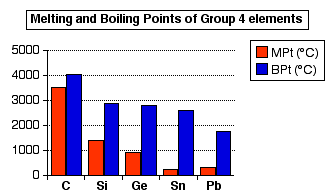 The Trend From Non Metal To Metal In Group 4 From chemguide.co.uk
The Trend From Non Metal To Metal In Group 4 From chemguide.co.uk
As the number of carbon atoms increases or the length of carbon-carbon chain increases the boiling point also increases. 462 234 1115. Carbon dioxide melts at -566 o C. Pure ethyl ethanoate boils at 73oC. The chemical elements of the periodic chart sorted by. Carbon oxide Flue gas Monoxide CAS No.
Here T BP is the boiling point elevation– the change in the boiling point that occurs when a solute dissolves in the solvent and k b is a proportionality constant known as the molal boiling point elevation constant for the solvent.
1560 626 306 Camphor. You can now roughly evaluate its boiling point. The liquid may then be filtered or decanted and distilled in clean dry Quickfit. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. It takes more energy to overcome the force of attraction and so the boiling point rises. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F.
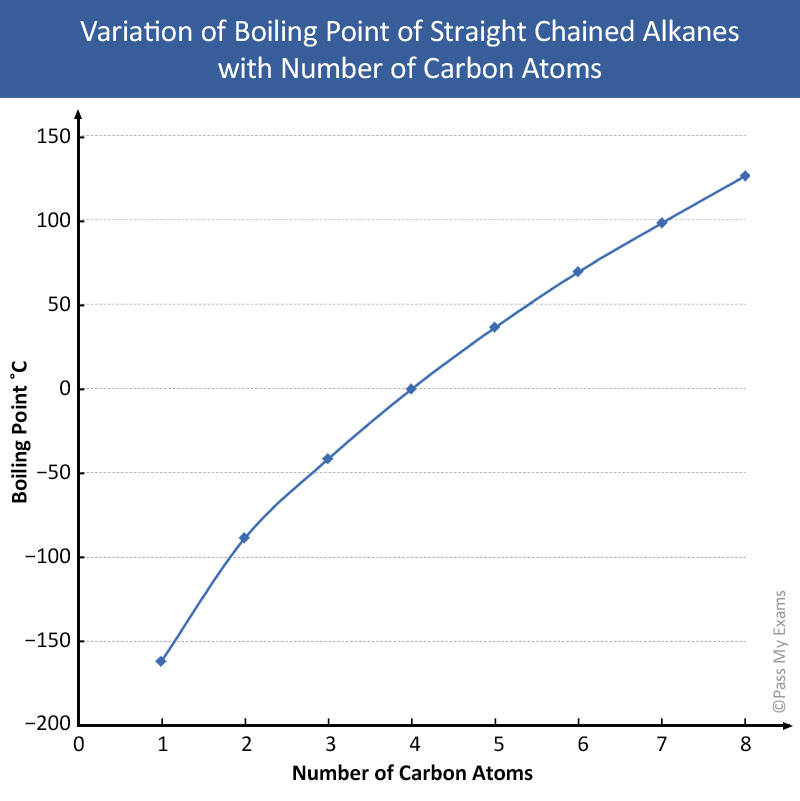 Source: passmyexams.co.uk
Source: passmyexams.co.uk
Propane CH3CH2CH3 or C3H8 CID 6334 - structure chemical names physical and chemical properties classification patents literature biological activities. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. DOT ID Guide. Branching decreases the boiling point. The chemical elements of the periodic chart sorted by.
 Source: researchgate.net
Source: researchgate.net
Carbon dioxide melts at -566 o C. A similar equation can be written to describe what happens to the freezing point or melting point of a solvent when a solute is added to the solvent. Boiling point C K b Ckgmol Freezing point C K f Ckgmol Data source. Melting point boiling point and vapor pressure of organic chemicals are estimated using a combination of techniques. Some fuels and their boiling points at atmospheric pressure.
Source: quora.com
1016 119 9202 168cryogenic liquid Formula. This is the answer. 1016 119 9202 168cryogenic liquid Formula. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. 1560 626 306 Camphor.
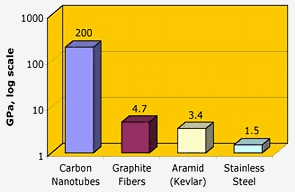 Source: chemicool.com
Source: chemicool.com
Just like how the strength of the bonds between atoms affect the Melting Point the boiling point depends on the heat energy required to create a transition from liquid to gaseous state. Boiling Point and Dipole-Dipole Interactions. 2040 595 179 40 K f. Enter 760 millimeters of mercury or 1013 hPa – units do not matter as the pressure value and 100 as the boiling point. DOT ID Guide.
 Source: researchgate.net
Source: researchgate.net
Some fuels and their boiling points at atmospheric pressure. Pure ethyl ethanoate boils at 73oC. This chemistry video tutorial explains the concepts behind the phase diagram of CO2 Carbon Dioxide and the phase diagram of water H2O. Take water for example. Just like how the strength of the bonds between atoms affect the Melting Point the boiling point depends on the heat energy required to create a transition from liquid to gaseous state.
 Source: britannica.com
Source: britannica.com
T FP -k. Van Der Waals radius. In all three states the same molecules of water H 2 O are present. As the liquid matter is heated further it eventually boils or vaporizes into a gas at the boiling point. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F.
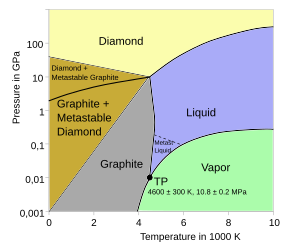 Source: en.wikipedia.org
Source: en.wikipedia.org
DOT ID Guide. 562 167 948 K b. Included is the subcooled liquid vapor pressure which is the vapor pressure a solid would have if it were liquid at room temperature. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F. Atomic number - Name alphabetically-269.
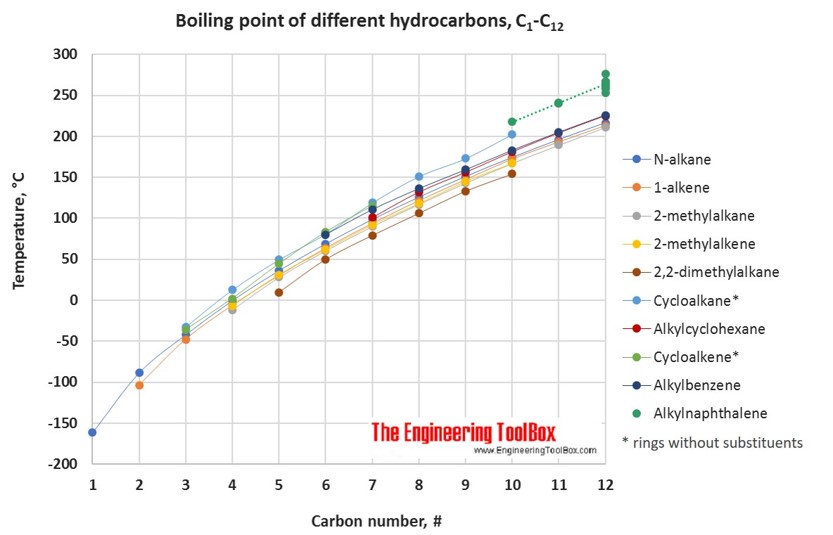 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
As the liquid matter is heated further it eventually boils or vaporizes into a gas at the boiling point. This makes it useful as a base. Take water for example. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
 Source: chemguide.co.uk
Source: chemguide.co.uk
2989 44 39 Acetic acid. Ionic radius of mercury. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. It is important in fate modeling. Calls for climate reparations reach boiling point in Glasgow talks.
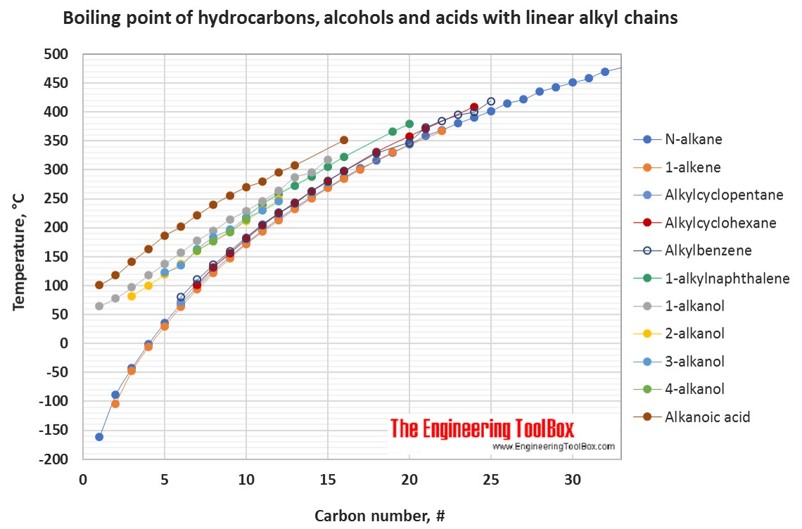 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
562 167 948 K b. 11 2021 at 851 pm. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. This is because the force of attraction between the molecules increases as the molecule gets longer and has more electrons. Calls for climate reparations reach boiling point in Glasgow talks.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.