Boiling point of calcium bromide
Home » datasheet » Boiling point of calcium bromideBoiling point of calcium bromide
Boiling Point Of Calcium Bromide. Electron configuration Ar3d 10 4s 2 4p 5. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. Boiling point F 345. Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by the liberation of heat.
 Our Products Bromine Noble Chemtech From brominenoblechemtech.com
Our Products Bromine Noble Chemtech From brominenoblechemtech.com
Sphere from calcium hydride before use. Modifications Pollutants removed from the list of hazardous air pollutants. Bromide-alinite in which bromide ions replace chloride has been made. A buffered acetic solution6a prepared with 330 g of sodium acetate 75 mL of glacial acetic acid and 35 mL of distilled water was used for hydrolyzing enolizable chiral a-fluoro ketimines. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O xAnhydrous MgCl 2 contains 255 elemental magnesium by mass. It has appreciable.
Calcium hydroxide dissolves in water to form its solution called lime water.
He liberated the element by passing chlorine through an aqueous solution of the. Modifications Pollutants removed from the list of hazardous air pollutants. I It is an endothermic reaction ii It is an exothermic reaction iii The. 1520 K Solubility in water. Add first dilute HNO 3 and then AgNO 3aq. It will give yellow precipitate of leadii bromidePbI 2.
 Source: t3db.ca
Source: t3db.ca
Determination of the enantiomeric excess by the use of tris3- heptafluoropropyl hydroxymethylene- -camphonato euro- piumII1 as the chiral shift. 312 at 20 C 68 F oxidation states. Electronic shell Ar 3d 10 4s 2 4p 5. Calcium silicate sulfate chloride Ca 4 SiO 4SO 4Cl 2 a derivative of alinite having an orthorhombic structure 173 is formed at only ca. I It is an endothermic reaction ii It is an exothermic reaction iii The.
 Source: byjus.com
Source: byjus.com
Energy of first ionisation. 4 - Includes organic compounds with more than one benzene ring and which have a boiling point greater than or equal to 100 ºC. Electronic shell Ar 3d 10 4s 2 4p 5. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant. 69 g100 mL methanol.
 Source: brominenoblechemtech.com
Source: brominenoblechemtech.com
It has appreciable. Determination of the enantiomeric excess by the use of tris3- heptafluoropropyl hydroxymethylene- -camphonato euro- piumII1 as the chiral shift. However its hydraulic activity has not yet been reported. Octahedral Thermochemistry Heat capacity C 70 Jmol K. Sphere from calcium hydride before use.

Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. 1250 C 2280 F. Sphere from calcium hydride before use. 69 g100 mL methanol. It will give yellow precipitate of leadii bromidePbI 2.

This process is called slaking of lime. Which among the following is are true about slaking of lime and the solution formed. Electron configuration Ar3d 10 4s 2 4p 5. Modifications Pollutants removed from the list of hazardous air pollutants. A buffered acetic solution6a prepared with 330 g of sodium acetate 75 mL of glacial acetic acid and 35 mL of distilled water was used for hydrolyzing enolizable chiral a-fluoro ketimines.
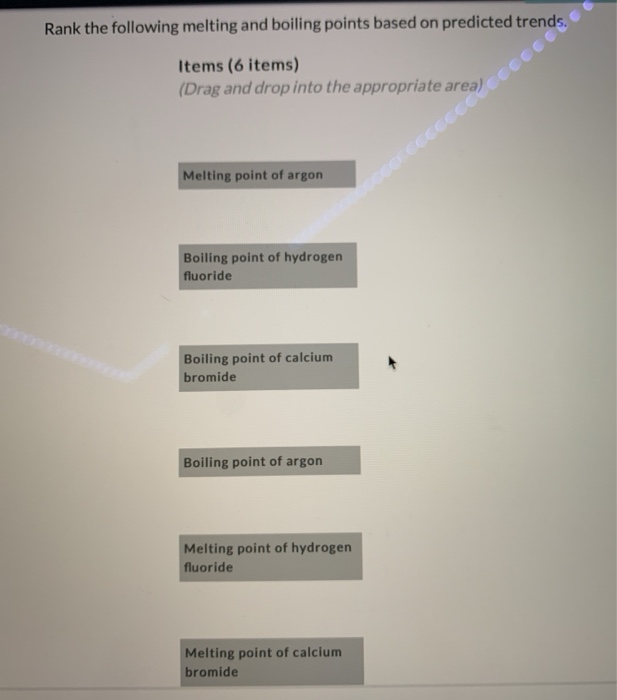 Source: chegg.com
Source: chegg.com
A buffered acetic solution6a prepared with 330 g of sodium acetate 75 mL of glacial acetic acid and 35 mL of distilled water was used for hydrolyzing enolizable chiral a-fluoro ketimines. Sphere from calcium hydride before use. It has appreciable. Which among the following is are true about slaking of lime and the solution formed. However its hydraulic activity has not yet been reported.
 Source: fishersci.se
Source: fishersci.se
59 C 138 F specific gravity. A buffered acetic solution6a prepared with 330 g of sodium acetate 75 mL of glacial acetic acid and 35 mL of distilled water was used for hydrolyzing enolizable chiral a-fluoro ketimines. Then cool the solution again it will deposit as a gold yellow powder. However its hydraulic activity has not yet been reported. At ambient temperature bromine is a brownish-red liquid.
 Source: chegg.com
Source: chegg.com
Add first dilute HNO 3 and then AgNO 3aq. I It is an endothermic reaction ii It is an exothermic reaction iii The. Boiling point F 345. It has a similarly colored vapor with an offensive and suffocating odor. Electronic shell Ar 3d 10 4s 2 4p 5.
Calcium silicate sulfate chloride Ca 4 SiO 4SO 4Cl 2 a derivative of alinite having an orthorhombic structure 173 is formed at only ca. 4 - Includes organic compounds with more than one benzene ring and which have a boiling point greater than or equal to 100 ºC. Electronic shell Ar 3d 10 4s 2 4p 5. It has appreciable. 69 g100 mL methanol.

A buffered acetic solution6a prepared with 330 g of sodium acetate 75 mL of glacial acetic acid and 35 mL of distilled water was used for hydrolyzing enolizable chiral a-fluoro ketimines. Energy of first ionisation. 1250 C 2280 F. This precipitate will melt when its boiling. Octahedral Thermochemistry Heat capacity C 70 Jmol K.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of calcium bromide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.