Boiling point of calcium
Home » datasheet » Boiling point of calciumBoiling point of calcium
Boiling Point Of Calcium. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. Just like how the strength of the bonds between atoms affect the Melting Point the boiling point depends on the heat energy required to create a transition from liquid to gaseous state. Freezing point depression is a colligative property of matter. The element is the fifth most abundant metal in the planets crust 41.
 Icon Of The Periodic Table Of Mendeleev Ca Chemical Element Ca With Atomic Mass Designation Electronic Configuration Melting Point Boiling Point And Name Text Calcium Stock Illustration Adobe Stock From stock.adobe.com
Icon Of The Periodic Table Of Mendeleev Ca Chemical Element Ca With Atomic Mass Designation Electronic Configuration Melting Point Boiling Point And Name Text Calcium Stock Illustration Adobe Stock From stock.adobe.com
Due to these two reasons metallic lattice of calcium is much greater than potassium. S Density g cm 3 154. Wear goggles and a lab coat. Colligative Properties of Matter. Melting point of Calcium is 842C. The chemical symbol for Calcium is Ca.
The occurrence of a dihydrate mineral Sinjarite and hexahydrate Antarcticite is very rare and is connected mainly with dry lakes and brines.
Calcium chloride removes water by forming a series of hydrates. Calcium Carbonate is the carbonic salt of calcium CaCO3. Calcium chloride CaCl 2 is a typical ionic halide and is a solid at room temperature. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. This type of hard water may be softened by using. Radius of calcium is smaller than potassium.
 Source: britannica.com
Source: britannica.com
Balanced formula has 6 positive charges 2 aluminum ions with a 3 charge and 6 negative charges 3 sulfate ions with -2 charge Mixtures Vs. Calcium chloride CaCl2 is a natural compound of calcium and chlorine derived from limestone. Interesting Facts about Calcium. Calciums symbol is Ca and has an atomic number 20 with atomic weight 40078amu. S Density g cm 3 154.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
1998-2013 Professor of Chemistry Iowa State University. Some of the best sources for calcium for our body include dairy products such as cheese yogurt and milk. The White Ring of Death right here. Calcium chloride CaCl2 is a natural compound of calcium and chlorine derived from limestone. Its physical and chemical properties are most.
 Source:
Source:
This must be because the boiling point of water falls with decreasing atmospheric pressure P atm. So for example if both calcium chloride CaCl 2 and sodium chloride NaCl completely dissolve in water the calcium chloride would lower the freezing point more than the sodium. This type of hard water may be softened by using. You can read more about Dwarf shrimp and Molting problems. It is a white solid although commercial samples appear yellow.
 Source: wdrfree.com
Source: wdrfree.com
The chemical properties of calcium include its melting point which is 8390 - 2C while its boiling point is 14840ºC with a valence of 2. Note that these points are associated with the standard atmospheric pressure. Its physical and chemical properties are most. The chemical properties of calcium include its melting point which is 8390 - 2C while its boiling point is 14840ºC with a valence of 2. So for example if both calcium chloride CaCl 2 and sodium chloride NaCl completely dissolve in water the calcium chloride would lower the freezing point more than the sodium.
 Source: material-properties.org
Source: material-properties.org
Calcium is an alkaline earth metal it is a reactive pale yellow metal that forms a dark oxide-nitride layer when exposed to air. 125 g100 mL 0 C 143 g100 mL 20 C 312 g100 mL 100 C Solubility in alcohol acetone. 2015present Senior Instructor II University of Oregon. That is why there must be a balance between Calcium and Magnesium ratio 31. Pricking the blunt end of an egg does not as is commonly thought prevent the shell from cracking.
 Source: materials.gelsonluz.com
Source: materials.gelsonluz.com
Colligative properties depend on the number of particles present not on the type of particles or their mass. Colligative properties depend on the number of particles present not on the type of particles or their mass. It was first isolated in 1808 in England when Sir Humphry Davy electrolyzed a mixture of lime and mercuric oxide. The point is that Magnesium keeps the calcium in a dissolved state and helps calcium absorption. It relieves pressure.
 Source: slideshare.net
Source: slideshare.net
The chemical symbol for Calcium is Ca. Clarke SI Mazzafro WJ. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. 2013-2014 Visiting Lecturer University of Oregon.
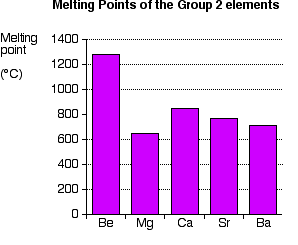 Source: chemguide.co.uk
Source: chemguide.co.uk
This is only achievable with the use of special industry or laboratory equipment. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Due to these two reasons metallic lattice of calcium is much greater than potassium. Clarke SI Mazzafro WJ.
 Source: stock.adobe.com
Source: stock.adobe.com
It was first isolated in 1808 in England when Sir Humphry Davy electrolyzed a mixture of lime and mercuric oxide. Wear goggles and a lab coat. Colligative properties depend on the number of particles present not on the type of particles or their mass. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. The occurrence of a dihydrate mineral Sinjarite and hexahydrate Antarcticite is very rare and is connected mainly with dry lakes and brines.
 Source: pinterest.ca
Source: pinterest.ca
2013-2014 Visiting Lecturer University of Oregon. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. Freezing point depression is a colligative property of matter. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Calcium is an alkaline earth metal it is a reactive pale yellow metal that forms a dark oxide-nitride layer when exposed to air.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of calcium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.