Boiling point of caffeine
Home » datasheet » Boiling point of caffeineBoiling point of caffeine
Boiling Point Of Caffeine. Influence of the Heating Rate on the Melting Point Measurement. Results depend strongly on the heating rate - the higher the. Removal of organic. The most prominent is that it reversibly blocks the action of.
 Caffeine 237 C Melting Point Standard Lgc Standards From lgcstandards.com
Caffeine 237 C Melting Point Standard Lgc Standards From lgcstandards.com
Research has been taken to extract it from natural source more economically. Brew 3-5 tea bags in two cups of boiling water in heat-proof pitcher. 1 Pa at 20 C Acidity pK a 774. It is a molecular solid with low conductivity. Serve with milk if desired and sweeten to taste. After removing trace amounts of water the low-boiling solvent is evaporated to yield the crude organic extracts.
It is weakly basic pK a of conjugate acid 06 requiring strong acid to protonate it.
The melting point of caffeine is 235C and the boiling point is 178C 4. Whenever the powder is added to boiling water the result is a bright green frothy drink that is naturally sweet. Caffeine has a molar mass of 19419gmol. Soluble in ethyl acetate or pyrrole. UV-vis λ max 280 nm Refractive index n D 1485 Thermochemistry Heat capacity C 389 J K 1 mol 1. It is a molecular solid with low conductivity.
 Source: researchgate.net
Source: researchgate.net
Caffeine had been widely used in the food and pharma industry. Herbal teas are naturally caffeine-free so caffeine is not an issue when consuming this type of tea. It contains almost double-fold of the concentration of caffeine. No natural isomers of caffeine exist. Most commercial brands of herbal teas are thought to be.
 Source: researchgate.net
Source: researchgate.net
Caffeine is a methylxanthine alkaloid found in the seeds nuts or leaves of a number of plants native to South America and East Asia that is structurally related to adenosine and acts primarily as an adenosine receptor antagonist with psychotropic and anti-inflammatory activities. LKT Labs C0221 Soluble in pyrrole THF-H2O mixture ethyl acetate Alfa Aesar A10431. The water temperature should be 160F - 180F well under the boiling point. Inhibits the action of phosphodiesterase an enzyme which inhibits cyclic adenosine monophosphate. Upon ingestion caffeine binds to adenosine receptors in the central nervous system CNS which inhibits.
 Source: lgcstandards.com
Source: lgcstandards.com
Pure anhydrous caffeine is a bitter-tasting white odorless powder with a melting point of 235238 C. Boiling point is in the range of 30 and 40 degree celsius while caffeines boiling point is 173 degree celsiusa lot higher than that of dichloromethane so the caffeine solution remained in the container. The most prominent is that it reversibly blocks the action of. Caffeine does not. On-Demand Webinar Good Melting Point Practice Back to Questions.
 Source: researchgate.net
Source: researchgate.net
Other common applications of liquid-liquid extractions involve. A second crop of caffeine may form in the filtrate as the solvent evaporates. It contains almost double-fold of the concentration of caffeine. If you dont have an electric kettle or thermometer handy an easy rule is 14 room temp. The dominant factor here is hydrogen bonding and the first table below documents the powerful intermolecular attraction that results from -O-H — O- hydrogen bonding in alcohols light blue columns.
 Source: semanticscholar.org
Source: semanticscholar.org
If you dont have an electric kettle or thermometer handy an easy rule is 14 room temp. Results depend strongly on the heating rate - the higher the. It is also moderately soluble in ethanol 15 g100 mL. Caffeine has no stereoisomers as there are no tetrahedral structures with all different substituents preventing a chiral center See Figure 1. The decaffeination process is initiated before coffee beans have been roasted and substances like solvents are removed before.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. There are several known mechanisms of action to explain the effects of caffeine. Statements regarding dietary supplements. Caffeine is a soft white crystal or powder with an intensely bitter taste when in its pure form. For a refreshing quart of iced tea.
 Source: researchgate.net
Source: researchgate.net
Slightly soluble in water solubility is increased by adding dilute acid. Decaf coffee does not have the negative side effects of caffeine. Depending on the strength of the brew pu-erh tea can contain 30100 mg of. Figure 1- The figure above is the 3D. Caffeine is a soft white crystal or powder with an intensely bitter taste when in its pure form.
 Source: dreamstime.com
Source: dreamstime.com
The dominant factor here is hydrogen bonding and the first table below documents the powerful intermolecular attraction that results from -O-H — O- hydrogen bonding in alcohols light blue columns. It is weakly basic pK a of conjugate acid 06 requiring strong acid to protonate it. Caffeine is a central nervous system CNS stimulant of the methylxanthine class. Most of the side effects of pu-erh tea come from its caffeine content. Isolation of caffeine from tea leaves.
 Source: en.wikipedia.org
Source: en.wikipedia.org
After air drying weigh each crop and record your caffeine recovered from tea. It is weakly basic pK a of conjugate acid 06 requiring strong acid to protonate it. It is odourless as there are no gas molecules being given off due to its solid state. Caffeine has a molar mass of 19419gmol. At the boiling temperature than at 30C.
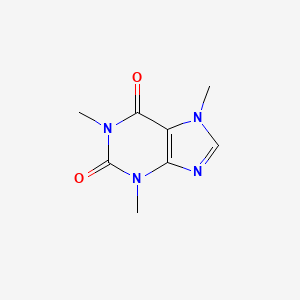
Removal of acid base and salt impurities. Most commercial brands of herbal teas are thought to be. It is also moderately soluble in ethanol 15 g100 mL. Brew in boiling water 3-5 minutes. Boiling point is in the range of 30 and 40 degree celsius while caffeines boiling point is 173 degree celsiusa lot higher than that of dichloromethane so the caffeine solution remained in the container.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of caffeine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.