Boiling point of butanol
Home » datasheet » Boiling point of butanolBoiling point of butanol
Boiling Point Of Butanol. The boiling point of n-butanol is 117 o C. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F. In mice after a single ip injection of 81 mmol tert-butanolkg body weight initial blood levels of 8 mmol took 8-9 hr for elimination blood- tert-butanol half-life was approximately 5 hr. This is significant in cooling the car since as soon as the water in the car radiator boilsit does not work anymore as a coolant.
 Amf From iea-amf.org
Amf From iea-amf.org
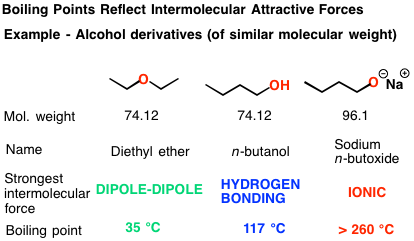
Caesium 40 o C 980. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. Near the boiling point of the solvent. N-butanol CH3CH2CH2CH2OH 8 n-pentanol CH3CH2CH2CH2CH2OH 2 n-hexanol CH3CH2CH2CH2CH2CH2OH 05 n-pentanol CH3CH2CH2CH2CH2CH2CH2OH 01 Compounds with six or more carbons for each polar group will not be very soluble in polar solvents but will be soluble in non. But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond. Ethane -886 o C 1316.
Exposure to an air concn of 50 ppm for 2 hr resulted in blood levels less than 008 mgl.
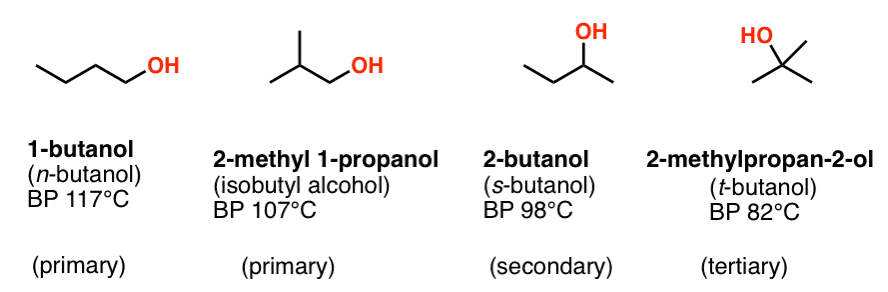
Making butanol from oil produces no such odour. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. 1-Butanol 20 o C 1262. We mentioned in the previous post that stronger intermolecular interactions increase the boiling and melting points but how exactly they affect the physical properties might be your next question. It is one of several isomers of amyl alcohol pentanol. Small amount of compound being purified should remain in solution at low temperatures.
But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond. Butanols only major disadvantages are its high flashpoint 35 C or 95 F toxicity note that toxicity levels exist but are not precisely confirmed and the fact that the fermentation process for renewable butanol emits a foul odour. Chlorobenzene 25 o C 1270. Making butanol from oil produces no such odour. N-butanol CH3CH2CH2CH2OH 8 n-pentanol CH3CH2CH2CH2CH2OH 2 n-hexanol CH3CH2CH2CH2CH2CH2OH 05 n-pentanol CH3CH2CH2CH2CH2CH2CH2OH 01 Compounds with six or more carbons for each polar group will not be very soluble in polar solvents but will be soluble in non.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. The freezing point depression aspectcan also be applied in daily life for example in raising the boiling point of the water that cools cars. Chlorobenzene 25 o C 1270. Cyclohexane 20 o C 1280. Antifreeze decreases the freezing point and increases the boiling point of thecoolant water in the car radiator.
 Source: iea-amf.org
Source: iea-amf.org
Chloroform 25 o C 984. Caesium 40 o C 980. Chlorobenzene 25 o C 1270. Cyclohexane 20 o C 1280. It is also known as isopentyl alcohol isopentanol or in the IUPAC recommended nomenclature 3-methyl-butan-1-olAn obsolete name for it was isobutyl carbinol.
 Source: iea-amf.org
Source: iea-amf.org
Isoamyl alcohol is an ingredient in the production of. It is one of several isomers of amyl alcohol pentanol. It is also known as isopentyl alcohol isopentanol or in the IUPAC recommended nomenclature 3-methyl-butan-1-olAn obsolete name for it was isobutyl carbinol. The intermolecular forces go in the order Ionic Hydrogen Bonding Dipole. N-butanol CH3CH2CH2CH2OH 8 n-pentanol CH3CH2CH2CH2CH2OH 2 n-hexanol CH3CH2CH2CH2CH2CH2OH 05 n-pentanol CH3CH2CH2CH2CH2CH2CH2OH 01 Compounds with six or more carbons for each polar group will not be very soluble in polar solvents but will be soluble in non.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Boiling Point and Dipole-Dipole Interactions. 1-Butanol 20 o C 1262. It is also known as isopentyl alcohol isopentanol or in the IUPAC recommended nomenclature 3-methyl-butan-1-olAn obsolete name for it was isobutyl carbinol. Small amount of compound being purified should remain in solution at low temperatures. Ethanol 20 o C 1159.
 Source: en.wikipedia.org
Source: en.wikipedia.org
But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond. But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond. Chlorobenzene 25 o C 1270. Caesium 40 o C 980. Ethane -886 o C 1316.
 Source: chemohollic.com
Source: chemohollic.com
Melting PointRange-89 C -1282 F Boiling PointRange 1176 C 2437 F Flash Point 35 C 95 F Method - Closed cup Evaporation Rate 046 Flammability solidgas Not applicable Flammability or explosive limits Upper 112 vol Lower 14 vol Vapor Pressure 67 mbar 20 C Vapor Density 26 Specific Gravity 0810. The intermolecular forces go in the order Ionic Hydrogen Bonding Dipole. Small amount of compound being purified should remain in solution at low temperatures. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. Chloroform 25 o C 984.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Chloroform 25 o C 984. Melting PointRange-89 C -1282 F Boiling PointRange 1176 C 2437 F Flash Point 35 C 95 F Method - Closed cup Evaporation Rate 046 Flammability solidgas Not applicable Flammability or explosive limits Upper 112 vol Lower 14 vol Vapor Pressure 67 mbar 20 C Vapor Density 26 Specific Gravity 0810. The intermolecular forces go in the order Ionic Hydrogen Bonding Dipole. Ethanol 20 o C 1159. The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure.
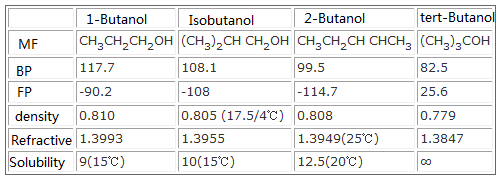 Source: chemicalbook.com
Source: chemicalbook.com
Ethanol 20 o C 1159. Making butanol from oil produces no such odour. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. Caesium 40 o C 980. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F.
 Source: iea-amf.org
Source: iea-amf.org
Cyclohexanol 25 o C 1465. Cyclohexanol 25 o C 1465. In mice after a single ip injection of 81 mmol tert-butanolkg body weight initial blood levels of 8 mmol took 8-9 hr for elimination blood- tert-butanol half-life was approximately 5 hr. Caesium 40 o C 980. Chloroform 25 o C 984.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of butanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.