Boiling point of benzene
Home » datasheet » Boiling point of benzeneBoiling point of benzene
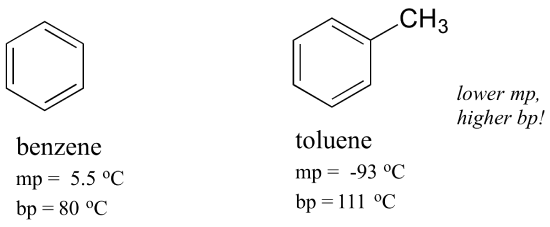
Boiling Point Of Benzene. Due to the cyclic. Note also that the boiling point for toluene is 111 o C well above the boiling point of benzene 80 o C. For hydrocarbons with the same carbon number the boiling point increases in the following order. 647 C 1485 F Boiling point of acetone.
 3 3 Melting Points And Boiling Points Introductory Organic Chemistry From openoregon.pressbooks.pub
3 3 Melting Points And Boiling Points Introductory Organic Chemistry From openoregon.pressbooks.pub
Boiling point of water. Benzene is an organic chemical compound with the molecular formula C 6 H 6. 7837 C 1731 F Boiling point of nitrogen. Two molecules went into making the dimer but only one item the dimer is present in solution. -1958 C -3204 F Boiling point of liquid helium. The key factor for the boiling point trend in this case is size toluene has one more carbon whereas for the melting point trend shape plays a much more important role.
-1958 C -3204 F Boiling point of liquid helium.
Take a capillary tube and close its one end by holding the end in the flame and rotate it for 2-3 minutes. The key factor for the boiling point trend in this case is size toluene has one more carbon whereas for the melting point trend shape plays a much more important role. 100 C 212 F Boiling point of water in Kelvin. 7837 C 1731 F Boiling point of methanol. When a solute is dissolved in a solvent the number. In order for a liquid to boil its vapor pressure needs to exceed ambient pressure which is harder to achieve once you add a nonvolatile component.
 Source: researchgate.net
Source: researchgate.net
Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Boiling point - the temperature at which a liquid turns into a gas. This makes sense when you consider that melting involves unpacking the molecules from their ordered array. 812 o C 801 o C 1253 o Ckgmol-1x02 kg x 11 o C02kg253 o Ckgmol-1 x 00869 moles. Vant Hoff must have been.
 Source: study.com
Source: study.com
Benzene is a liquid at standard conditions. Boiling point - the temperature at which a liquid turns into a gas. Benzene is an organic chemical compound with the molecular formula C 6 H 6. In order for a liquid to boil its vapor pressure needs to exceed ambient pressure which is harder to achieve once you add a nonvolatile component. 100 C 212 F Boiling point of water in Kelvin.

Dip the capillary tube into the liquid in the fusion tube keeping the sealed end up. 812 o C 801 o C 1253 o Ckgmol-1x02 kg x 11 o C02kg253 o Ckgmol-1 x 00869 moles. Note also that the boiling point for toluene is 111 o C well above the boiling point of benzene 80 o C. 8191C 802C 171C Δ T b. The mole fraction of component 1 in the mixture can be represented by the symbol x 1.
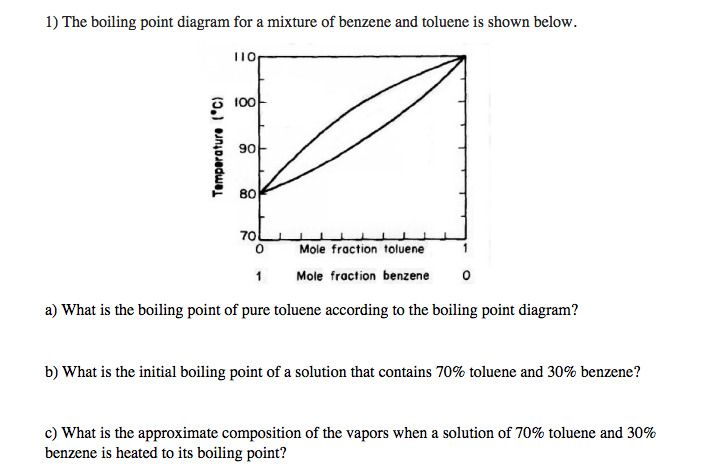 Source: chegg.com
Source: chegg.com
For hydrocarbons with the same carbon number the boiling point increases in the following order. That concentration is the number of moles per kilogram of benzene but the solution used only 300 grams of the. And then when he got to the right answer I think the joy of figuring it out must have been mixed with some relief. 56 C 1328 F Boiling point of alcohol. 7837 C 1731 F Boiling point of nitrogen.
 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
For hydrocarbons with the same carbon number the boiling point increases in the following order. From the boiling point elevation formula the following relationship can be obtained. 8191C 802C 171C Δ T b. However if heated it becomes a gas and when cooled it becomes a solid. Boiling point of water.
 Source: youtube.com
Source: youtube.com
812 o C 801 o C 1253 o Ckgmol-1x02 kg x 11 o C02kg253 o Ckgmol-1 x 00869 moles. The curve between the critical point and the triple point shows the benzene boiling point with changes in pressure. When the temperature of a liquid is below its boiling point we can assume that the only molecules that can escape from the liquid to form a gas are those that lie near the surface of the liquid. The acetic acid dimerized in the benzene and formed CH 3 COOH 2. Benzene is an organic chemical compound with the molecular formula C 6 H 6.
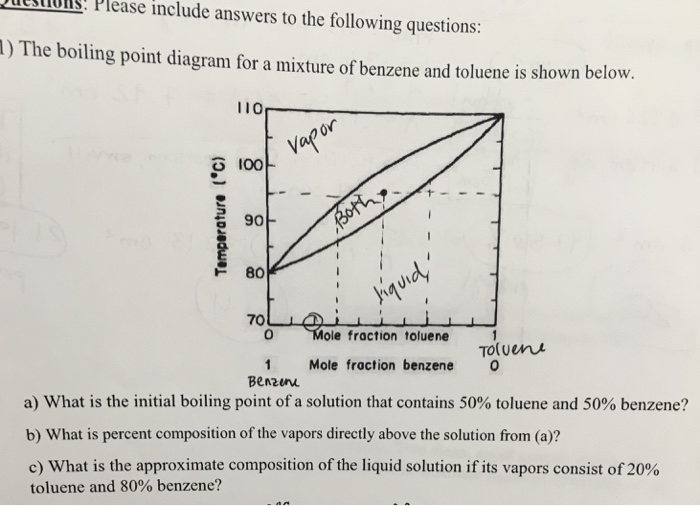
7837 C 1731 F Boiling point of nitrogen. 56 C 1328 F Boiling point of alcohol. 647 C 1485 F Boiling point of acetone. -1958 C -3204 F Boiling point of liquid helium. 812 o C 801 o C 1253 o Ckgmol-1x02 kg x 11 o C02kg253 o Ckgmol-1 x 00869 moles.
 Source: ddbst.com
Source: ddbst.com
The acetic acid dimerized in the benzene and formed CH 3 COOH 2. Take a capillary tube and close its one end by holding the end in the flame and rotate it for 2-3 minutes. Vant Hoff must have been. Transfer a few mL of benzene in the fusion tube. 7837 C 1731 F Boiling point of methanol.
 Source: researchgate.net
Source: researchgate.net
-269 C -452 F. When the temperature of a liquid is below its boiling point we can assume that the only molecules that can escape from the liquid to form a gas are those that lie near the surface of the liquid. The curve between the critical point and the triple point shows the benzene boiling point with changes in pressure. For the boiling point of pure benzene the boiling point elevation is. When a solute is added to the solvent some of the solute molecules occupy the space near the surface of the liquid as shown in the figure below.
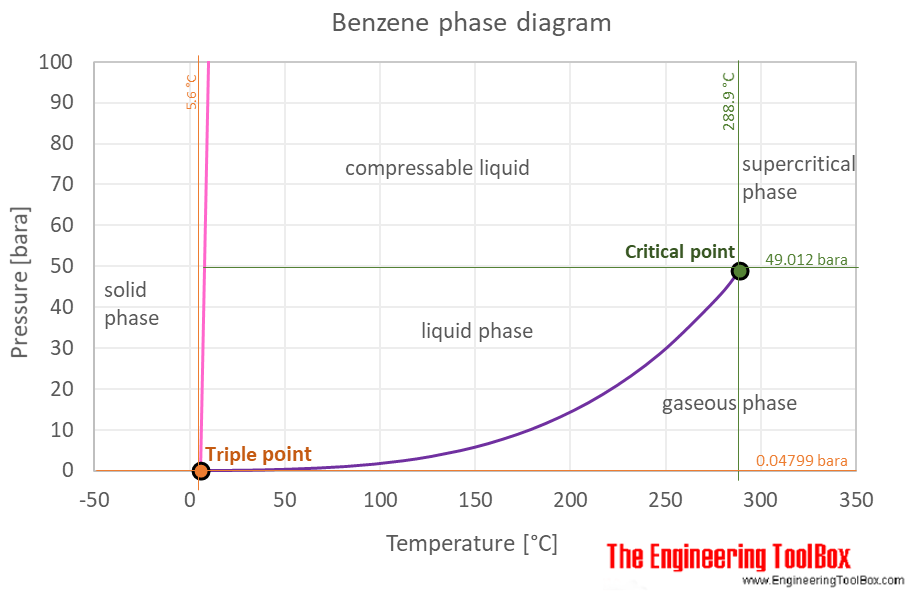 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It also shows. Transfer a few mL of benzene in the fusion tube. 8191C 802C 171C Δ T b. That concentration is the number of moles per kilogram of benzene but the solution used only 300 grams of the. 100 C 212 F Boiling point of water in Kelvin.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of benzene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.