Boiling point of aq hcl
Home » datasheet » Boiling point of aq hclBoiling point of aq hcl
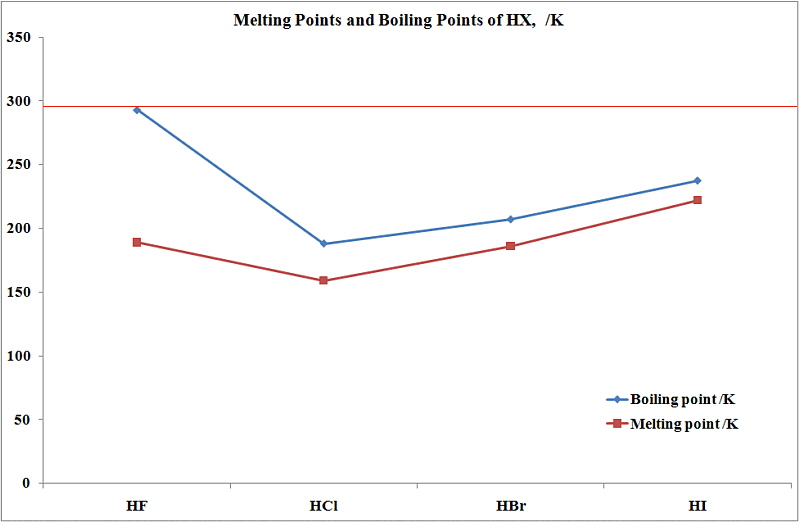
Boiling Point Of Aq Hcl. Variations of the melting point blue curve and boiling points red in the first row transition metals. Fluorine has the highest e-neg SO HF will experience the strongest H bonding and needs the most energy to weaken the imf. Tungsten has the highest melting point where as silver has low boiling point. CH 3 CH 2 Cl or C 6 H 5 CH 2 Cl 47.
 Hydrochloric Acid Wikipedia From en.wikipedia.org
Hydrochloric Acid Wikipedia From en.wikipedia.org
Sodium and potassium have low melting points. 3 remove the supernatant and. 125 mL of 120 M HCl solution is diluted with water to a final volume of 100 LWhat is the molarity of the diluted solution. Tungsten has the highest melting point where as silver has low boiling point. Fluorine has the highest e-neg SO HF will experience the strongest H bonding and needs the most energy to weaken the imf. Which of the compounds will react faster in S N 1 reaction with the OH ion.
3 remove the supernatant and.
HF NH3 H2O WHY. When you have completed every question that you desire click the MARK TEST button after the last exerciseA new page will appear showing your correct and incorrect responses. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in. Questions left blank are not counted against you. This includes correcting mistakes quickly and. Pyridoxine hydrochloride is converted into the active form pyridoxal 5-phosphate PLP an essential cofactor in many enzymatic activities including synthesis of amino acids neurotransmitters and sphingolipids.

Levels of nitrate nitrite and S-nitrosothiols were measured in plasma saliva and urine before. Dilute HClaq acidic H2Saq CuSs Cu2aq Ca2aq Ca2aq Agaq Cu2aq Ca2aq NH4CO3aq CaCO3s The above flow chart translates to the following steps. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in. Out of o-and p-dibromobenzene which one has higher melting point and why. The authors tested if ingestion of inorganic nitrate would affect the salivary and systemic levels of nitrite and S-nitrosothiols both considered to be circulating storage pools for NO.

1998-2013 Professor of Chemistry Iowa State University. 1 add dilute HCl to precipitate AgCl from the mixture. TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in. When you have completed every question that you desire click the MARK TEST button after the last exerciseA new page will appear showing your correct and incorrect responses. 2 centrifuge sample to yield a solid pellet of AgCl and a supernatant the solution that remains above the pellet after centrifugation.
 Source: sciencedirect.com
Source: sciencedirect.com
Ability of aqueous solution to conduct electricity. If these colourless gases are allowed to mix a thick white smoke of solid ammonium chloride is formed. Other chemical properties include. 2 centrifuge sample to yield a solid pellet of AgCl and a supernatant the solution that remains above the pellet after centrifugation. And boil The End Chemical Bond A bond results from the attraction of nuclei for electrons All atoms trying to achieve a stable octet IN OTHER WORDS the p in one nucleus are attracted to the e.
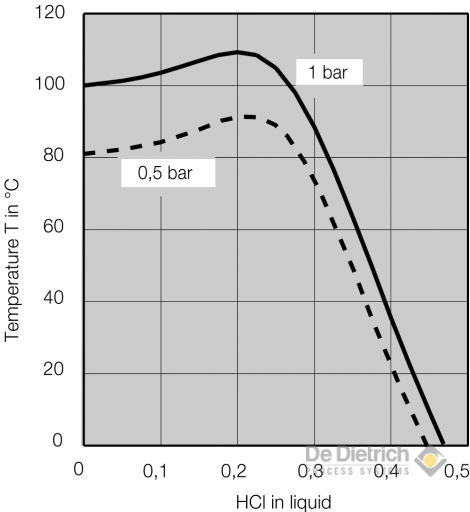 Source: dedietrich.com
Source: dedietrich.com
Out of o-and p-dibromobenzene which one has higher melting point and why. NH 3 HCl NH 4 Cl. Which substance has the highest boiling point. Why iodoform has appreciable antiseptic property. The University of Utah on Instagram.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The forces of attraction between these diatomic molecules is quite low so they are easily weakened by heat resulting in a low melting point and low boiling point. 1 add dilute HCl to precipitate AgCl from the mixture. We would like to show you a description here but the site wont allow us. This vitamin is essential to red blood cell nervous system and immune systems. Metals have high melting and boiling point.
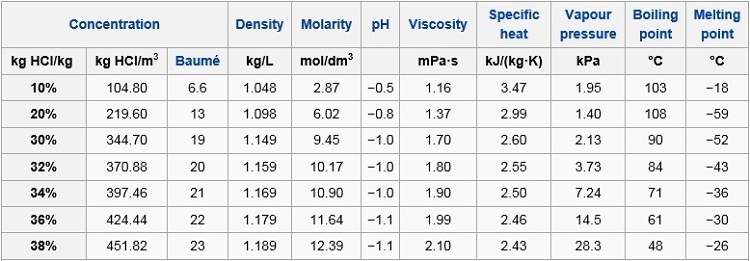 Source: hydro-land.com
Source: hydro-land.com
3 pts a 375 M b 150 M c 0185 M d 0015 M 23What is the molarity of an NaCl solution prepared by dissolving 93 g of NaCl in 350 mL of. Since Arts Bash can. The reaction between ammonia and hydrogen chloride. 2015present Senior Instructor II University of Oregon. CH 3 CH 2 Cl or C 6 H 5 CH 2 Cl 47.

1 add dilute HCl to precipitate AgCl from the mixture. The forces of attraction between these diatomic molecules is quite low so they are easily weakened by heat resulting in a low melting point and low boiling point. In other applications it is mostly obsolete and has been. Chlorine exists as a diatomic molecule. After sodium chlorate it is the second most common chlorate in industrial use.
 Source: msrblog.com
Source: msrblog.com
TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in. And boil The End Chemical Bond A bond results from the attraction of nuclei for electrons All atoms trying to achieve a stable octet IN OTHER WORDS the p in one nucleus are attracted to the e. Other chemical properties include. Melting and Boiling Points. Answer the following to the best of your ability.
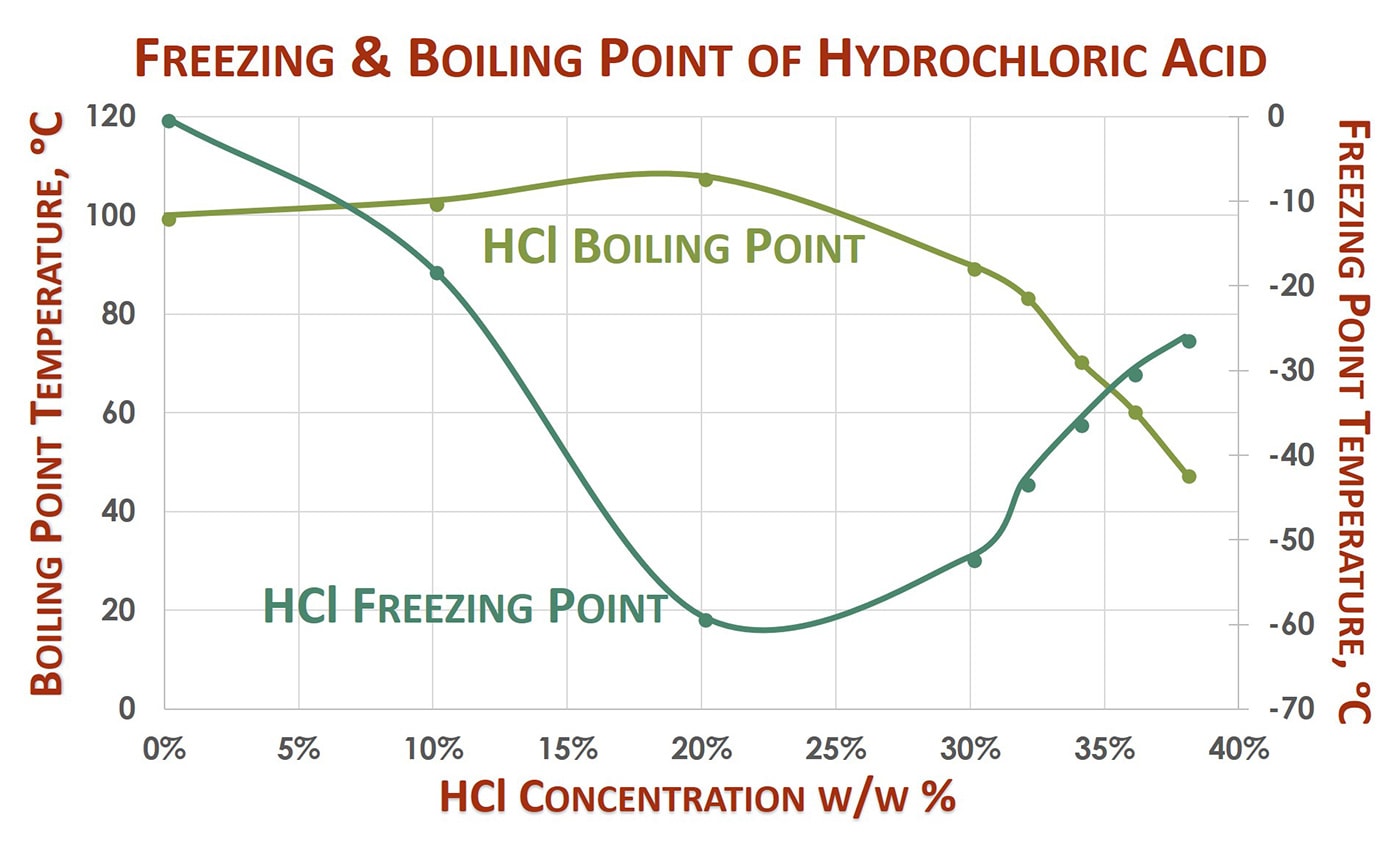 Source: protank.com
Source: protank.com
Why iodoform has appreciable antiseptic property. Which substance has the highest boiling point. Metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Chlorine exists as a diatomic molecule. Pyridoxine Hydrochloride is the hydrochloride salt form of pyridoxine a water-soluble vitamin B.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
Ability of aqueous solution to conduct electricity. This vitamin is essential to red blood cell nervous system and immune systems. Sodium and potassium have low melting points. The best way of producing a strong reducing agent is to pass an electric. After sodium chlorate it is the second most common chlorate in industrial use.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of aq hcl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.