Boiling point of alcohol
Home » datasheet » Boiling point of alcoholBoiling point of alcohol
Boiling Point Of Alcohol. At the boiling point molecules anywhere in the liquid may be vaporized. The boiling point decreases as atmospheric pressure decreases so it will be slightly lower unless you are at sea level. Calcium chloride removes water by forming a series of hydrates. The reverse reaction converting a gas to a liquid is called condensation.
2 Properties Of Alcohols Alcohol Carboxylic Acid And Esters From sites.google.com
The formation of large. Here is a look at the boiling point of different types of alcohol. A liquid boils when its vapour pressure is equal to the atmospheric pressure. This example illustrates the significance of bond strength in general and hydrogen bonding specifically as a determinant of volatility of a molecule. Whenever a nonvolatile chemical is dissolved in a liquid the increased number of particles in the liquid causes it to boil at a higher temperature. This in turn will affect the boiling point of both water and alcohol due to the weather conditions at any given time.
Here is a look at the boiling point of different types of alcohol.
Azertropes are Liquid Mixtures. Azertropes are Liquid Mixtures. The boiling point of organic compounds can give important information about their physical properties and structural characteristics. Set within the confines of an increasingly claustrophobic location director Philip Barantinis film succeeds because it understands the limitations of shooting in this manner using them to its advantage rather than trying to expand upon what something in this style could achieve. Boiling point helps identify and characterise a compound. Boiling Points and Vapor Pressure Background 2 As a very general rule of thumb the boiling point of many liquids will drop about 05C for a 10mmHg decrease in pressure when operating in the.
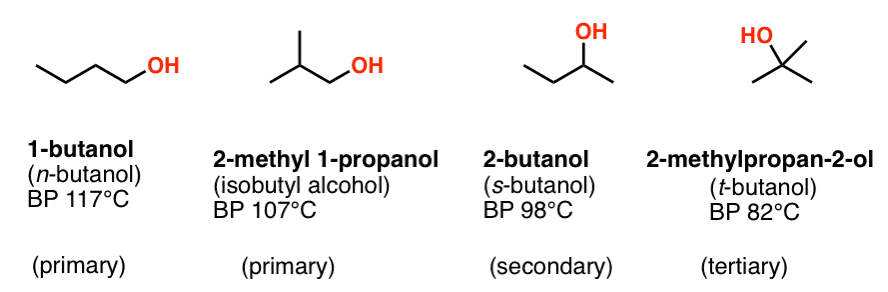 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
100 vol water dissolve 65 vol at 178 C 753 mm Hg. The boiling points of the alcohols increase as the number of carbon atoms increases. 100 vol benzene dissolve 1452 vol at 215 C 757 mm Hg. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The formation of large.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The boiling point of alcohol depends on which type of alcohol youre using as well as the atmospheric pressure. The boiling point of an alcohol is always much higher than that of the alkane with the same number of carbon atoms. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compound. 100 vol benzene dissolve 1452 vol at 215 C 757 mm Hg. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher.
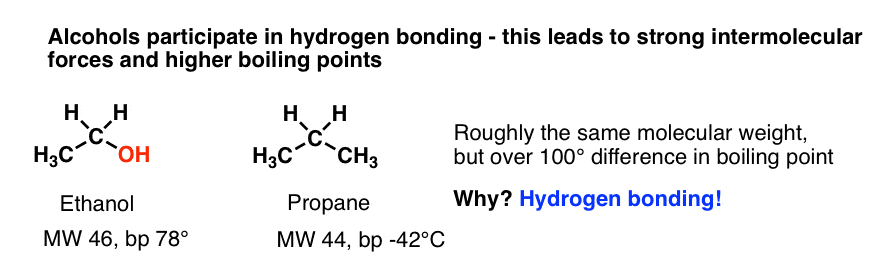 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The reverse reaction converting a gas to a liquid is called condensation. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation. Acid alcohol ester water. Boiling Point is among the finer examples of the one-take movie.
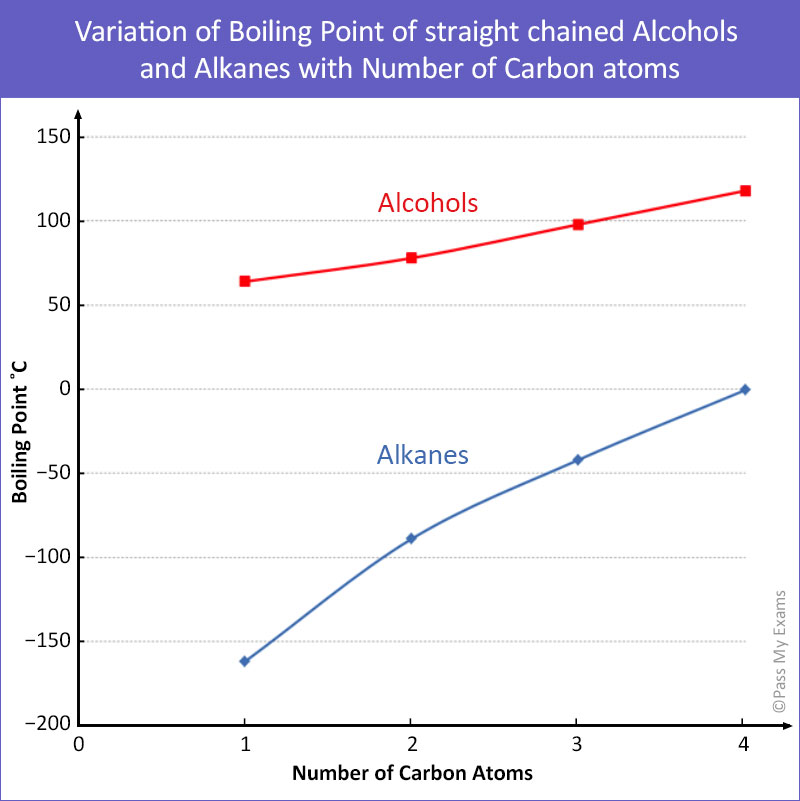 Source: passmyexams.co.uk
Source: passmyexams.co.uk
Thus it becomes the rare instance where this. Azeotropes occur when fraction of the liquids cannot be altered by distillation. 100 vol water dissolve 65 vol at 178 C 753 mm Hg. Their mixture can either have a higher or lower boiling point than either of the components individually. The boiling point of organic compounds can give important information about their physical properties and structural characteristics.
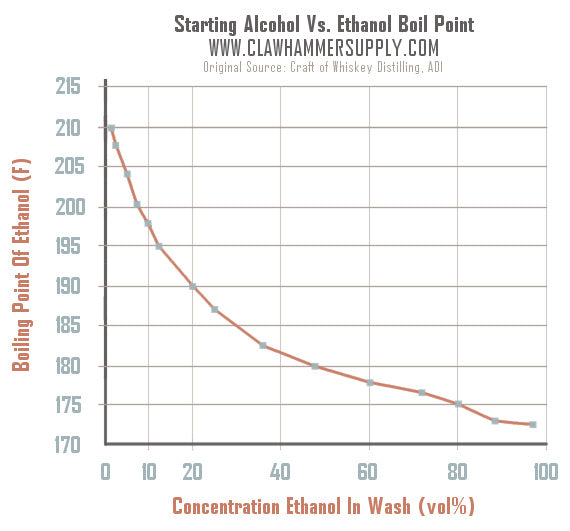 Source: clawhammersupply.com
Source: clawhammersupply.com
So while providing freeze protection and an increased boiling point ethylene glycol lowers the specific heat capacity of water mixtures relative to pure water. Boiling Point is among the finer examples of the one-take movie. Melting Point and Boiling point- Melting point is a characteristic property of solid crystalline substances. The boiling point at atmospheric pressure. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compound.
 Source: researchgate.net
Source: researchgate.net
The boiling point decreases as atmospheric pressure decreases so it will be slightly lower unless you are at sea level. The reverse reaction converting a gas to a liquid is called condensation. Azeotropes occur when fraction of the liquids cannot be altered by distillation. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point of a substance is the temperature at which it changes state from liquid to gas throughout the bulk of the liquid.
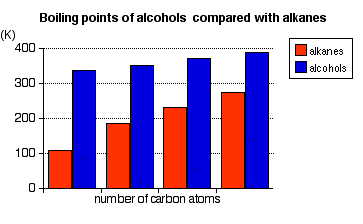 Source: chemguide.co.uk
Source: chemguide.co.uk
The boiling point of organic compounds can give important information about their physical properties and structural characteristics. You can think of milk as water that contains salts sugars fats and other molecules. The boiling point of alcohol depends on which type of alcohol youre using as well as the atmospheric pressure. This example illustrates the significance of bond strength in general and hydrogen bonding specifically as a determinant of volatility of a molecule. The boiling point at atmospheric pressure.
 Source: thoughtco.com
Source: thoughtco.com
Thus it becomes the rare instance where this. At the boiling point molecules anywhere in the liquid may be vaporized. Set within the confines of an increasingly claustrophobic location director Philip Barantinis film succeeds because it understands the limitations of shooting in this manner using them to its advantage rather than trying to expand upon what something in this style could achieve. 100 vol absolute alcohol dissolve 790 vol at 166 C 754 mm Hg. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compound.
 Source: researchgate.net
Source: researchgate.net
Vapour pressure is determined by the kinetic energy of a molecule. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation. You can think of milk as water that contains salts sugars fats and other molecules. The reverse reaction converting a gas to a liquid is called condensation. The boiling point of organic compounds can give important information about their physical properties and structural characteristics.
Source: sites.google.com
Vapour pressure is determined by the kinetic energy of a molecule. The reverse reaction converting a gas to a liquid is called condensation. It is the temperature at which the solid phase changes to the liquid phaseThis is the point at which both liquid and solid phases exist at equilibriumVisit BYJUS to learn more about the Principle Detailed Explanation Videos and FAQs of melting point and Boiling point. 100 vol benzene dissolve 1452 vol at 215 C 757 mm Hg. It also reacts with alcohols to form similar compounds and can thus remove unchanged ethanol in.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of alcohol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.