Boiling point of acetonitrile
Home » datasheet » Boiling point of acetonitrileBoiling point of acetonitrile
Boiling Point Of Acetonitrile. The values in the table below except as noted have been extracted from online and hardbound compilations. Ethanol must have stronger intermolecular attraction based on its higher boiling point. 1 mmHg 300C Structure Crystal structure. Properties of Organic Solvents.
 Acetonitrile Hazards Formula Properties And Uses Free Chemistry Online From freechemistryonline.com
Acetonitrile Hazards Formula Properties And Uses Free Chemistry Online From freechemistryonline.com
Class IB Flash point below 73 F boiling point at or above 100 F. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. 787 K Solubility in water. Properties of Organic Solvents. Should be stored in approved flammable. Ethanol has a higher boiling point because of greater London dispersion force c.
Hydrolyses Solubility in other solvents 0222 g 100g CS 2 at 17 C Insoluble in C 6 H 6 Insoluble in hot xylene Insoluble in hot anisole.
1 mmHg 300C Structure Crystal structure. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Properties of Organic Solvents. 514 C 957 F. The values in the table below except as noted have been extracted from online and hardbound compilations. 14 mw86 has a boiling point of 68º.

Ethanol has a higher boiling point because of greater London dispersion force c. LD 50 median dose 389. Methyl ethyl ketone. Formic acid is a colorless liquid having a pungent penetrating odor at room temperature comparable to the related acetic acidIt is miscible with water and most polar organic solvents and is somewhat soluble in hydrocarbonsIn hydrocarbons and in the vapor phase it consists of hydrogen-bonded dimers rather than individual molecules. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003.
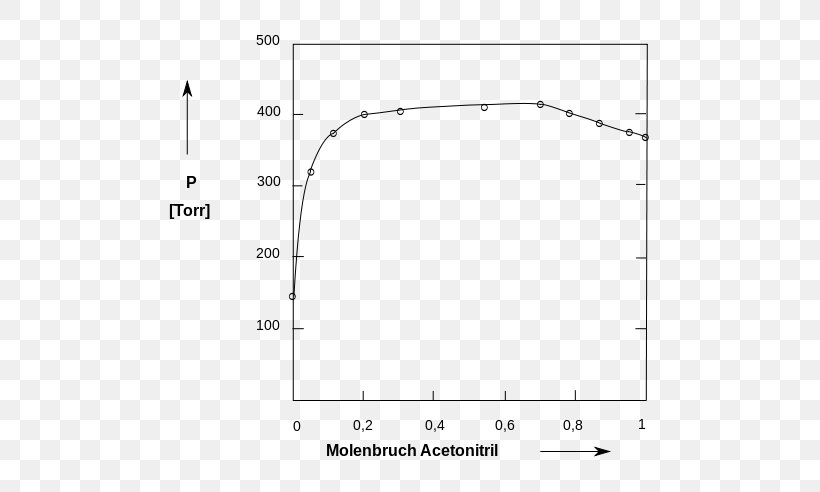 Source: favpng.com
Source: favpng.com
Naphthalene is a white volatile solid polycyclic hydrocarbon with a strong mothball odor. Naphthalene is a white volatile solid polycyclic hydrocarbon with a strong mothball odor. The values in the table below except as noted have been extracted from online and hardbound compilations. Class IB Flash point below 73 F boiling point at or above 100 F. Methyl ethyl ketone.

Owing to its tendency to hydrogen-bond gaseous formic. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Both hexane and. Class IB Flash point below 73 F boiling point at or above 100 F. Mark each of the following statements as TRUE or FALSE.
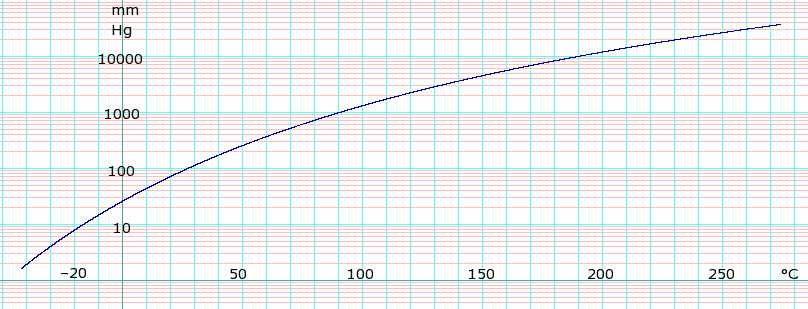 Source: en.wikipedia.org
Source: en.wikipedia.org
Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. 1 mmHg 300C Structure Crystal structure. Mark each of the following statements as TRUE or FALSE. Should be stored in approved flammable.
 Source: researchgate.net
Source: researchgate.net
Should be stored in approved flammable. Properties of Organic Solvents. Should be stored in approved flammable. 787 K Solubility in water. 1 mmHg 300C Structure Crystal structure.

Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride but is also used in moth repellentsExposure to naphthalene is associated with hemolytic anemia damage to the liver and neurological system cataracts and retinal hemorrhage. Ethanol CH 3CH 2OH mw46 has a boiling point of 78º. 514 C 957 F. Both hexane and. Owing to its tendency to hydrogen-bond gaseous formic.

Ethanol CH 3CH 2OH mw46 has a boiling point of 78º. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Ethanol must have stronger intermolecular attraction based on its higher boiling point. Ethanol CH 3CH 2OH mw46 has a boiling point of 78º. Class IB Flash point below 73 F boiling point at or above 100 F.
 Source: freechemistryonline.com
Source: freechemistryonline.com
Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. Naphthalene is a white volatile solid polycyclic hydrocarbon with a strong mothball odor. Both hexane and. Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride but is also used in moth repellentsExposure to naphthalene is associated with hemolytic anemia damage to the liver and neurological system cataracts and retinal hemorrhage. Formic acid is a colorless liquid having a pungent penetrating odor at room temperature comparable to the related acetic acidIt is miscible with water and most polar organic solvents and is somewhat soluble in hydrocarbonsIn hydrocarbons and in the vapor phase it consists of hydrogen-bonded dimers rather than individual molecules.
 Source: biopharma.co.uk
Source: biopharma.co.uk
Ethanol has a higher boiling point because of greater London dispersion force c. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. 14 mw86 has a boiling point of 68º. Both hexane and. Properties of Organic Solvents.
 Source: researchgate.net
Source: researchgate.net
Naphthalene is obtained from either coal tar or petroleum distillation and is primarily used to manufacture phthalic anhydride but is also used in moth repellentsExposure to naphthalene is associated with hemolytic anemia damage to the liver and neurological system cataracts and retinal hemorrhage. Hydrolyses Solubility in other solvents 0222 g 100g CS 2 at 17 C Insoluble in C 6 H 6 Insoluble in hot xylene Insoluble in hot anisole. Formic acid is a colorless liquid having a pungent penetrating odor at room temperature comparable to the related acetic acidIt is miscible with water and most polar organic solvents and is somewhat soluble in hydrocarbonsIn hydrocarbons and in the vapor phase it consists of hydrogen-bonded dimers rather than individual molecules. Should be stored in approved flammable. 787 K Solubility in water.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of acetonitrile by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.