Boiling point of acetone
Home » datasheet » Boiling point of acetoneBoiling point of acetone
Boiling Point Of Acetone. Very soluble in acetone diethyl ether ethanol. 101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure. Acetone is a liquid at standard conditions. 500 ppm Vacated TWA.
 Vapor Pressure Of Acetone From Dortmund Data Bank From ddbst.com
Vapor Pressure Of Acetone From Dortmund Data Bank From ddbst.com
If we know the boiling point of the substance at some specific pressure tables usually give the value under the so-called normal pressure ie. Boiling Point 133º F Melting Point -1372º F Specific Gravity 079 Molecular Weight gmole 5808 pH 7 Odor Sweet pungent Odor Threshold 62 ppm Vapor Pressure mm Hg 20º C 181 Solubility in Water Complete Volatile wt 100. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Above enter a boiling point to calculate the pressure. Heat of evaporation equals about kJmol Any pressure unit can be used. What is pure acetone.
Boiling Point 133º F Melting Point -1372º F Specific Gravity 079 Molecular Weight gmole 5808 pH 7 Odor Sweet pungent Odor Threshold 62 ppm Vapor Pressure mm Hg 20º C 181 Solubility in Water Complete Volatile wt 100.
If we know the boiling point of the substance at some specific pressure tables usually give the value under the so-called normal pressure ie. It is also called dimethyl ketone 2-propanone and beta-ketopropane. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Fire and Explosion Data Flash Point 14 Flammable Limits in Air By Volume. Acetone is a manufactured chemical that is also found naturally in the environment. 28 mmHg 20 C Magnetic susceptibility χ 689610 6 cm 3 mol Viscosity.
 Source: researchgate.net
Source: researchgate.net
However at low temperature andor very high pressures it becomes a solid. 500 ppm Vacated TWA. It is a colorless liquid with a distinct smell and taste. The result will be displayed in the same units. However at low temperature andor very high pressures it becomes a solid.
 Source: clutchprep.com
Source: clutchprep.com
Boiling Point 133º F Melting Point -1372º F Specific Gravity 079 Molecular Weight gmole 5808 pH 7 Odor Sweet pungent Odor Threshold 62 ppm Vapor Pressure mm Hg 20º C 181 Solubility in Water Complete Volatile wt 100. Heat of evaporation equals about kJmol Any pressure unit can be used. Acetone occurs naturally in the human body as a byproduct of metabolism. However at low temperature andor very high pressures it becomes a solid. Very soluble in acetone diethyl ether ethanol.
 Source: ddbst.com
Source: ddbst.com
The phase diagram for acetone shows the phase behavior with changes in temperature and pressure. The relationship between pressure change and temperature change during evaporation in general. As a solvent acetone is frequently incorporated in other solvent systems or blends used in the formulation of lacquers for automotive and furniture finishes for example. It also shows. Fire and Explosion Data Flash Point 14 Flammable Limits in Air By Volume.
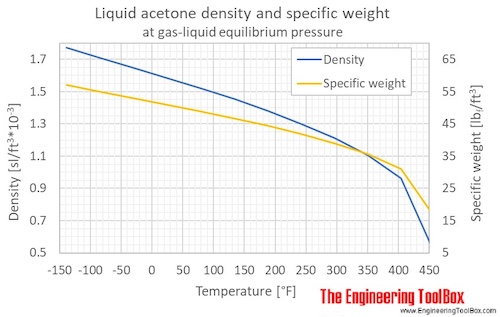 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The phase diagram for acetone shows the phase behavior with changes in temperature and pressure. Melting PointRange-95 C -139 F Boiling PointRange 56 C 1328 F Flash Point-20 C -4 F Method - CC closed cup Evaporation Rate 56 Butyl Acetate 10 Flammability solidgas Not applicable Flammability or explosive limits Upper 128 vol Lower 25 vol Component ACGIH TLV OSHA PEL NIOSH IDLH Mexico OEL TWA Acetone TWA. Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. 101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure. Slightly soluble 28 gL Solubility.

101325 hPa and enthalpy of vaporization molar heat of evaporation then we can estimate the boiling point under another selected pressure. The relationship between pressure change and temperature change during evaporation in general. The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. Acetone is also a primary ingredient in many nail polish removers. Heat of evaporation equals about kJmol Any pressure unit can be used.
 Source: en.wikipedia.org
Source: en.wikipedia.org
What is pure acetone. 4042 K Solubility in water. Below enter the boiling point under this pressure C C Above enter a pressure to calculate the boiling point. Heat of evaporation equals about kJmol Any pressure unit can be used. 28 mmHg 20 C Magnetic susceptibility χ 689610 6 cm 3 mol Viscosity.
 Source: mt.com
Source: mt.com
The phase diagram for acetone shows the phase behavior with changes in temperature and pressure. 28 mmHg 20 C Magnetic susceptibility χ 689610 6 cm 3 mol Viscosity. Some fuels and their boiling points at atmospheric pressure. Boiling Point 133º F Melting Point -1372º F Specific Gravity 079 Molecular Weight gmole 5808 pH 7 Odor Sweet pungent Odor Threshold 62 ppm Vapor Pressure mm Hg 20º C 181 Solubility in Water Complete Volatile wt 100. 3692 mPas Thermochemistry Heat capacity C 2382 JgK Std enthalpy of formation Δ f H 298 3564 kJmol liquid 3007 kJ.
 Source: clutchprep.com
Source: clutchprep.com
Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. Acetone is used to make plastic fibers drugs and other chemicals. Heat of evaporation equals about kJmol Any pressure unit can be used. What is pure acetone.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. 4042 K Solubility in water. Slightly soluble 28 gL Solubility. Sodium iodide chemical formula NaI is an ionic compound formed from the chemical reaction of sodium metal and iodineUnder standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal latticeIt is used mainly as a nutritional supplement and in organic chemistryIt is produced industrially as the salt formed when. 1311 C 2680 F.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It is a colorless liquid with a distinct smell and taste. Acetone MSDS Page 2 of 2 Rev. The phase diagram for acetone shows the phase behavior with changes in temperature and pressure. 500 ppm Vacated TWA. 1311 C 2680 F.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of acetone by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.