Boiling point of acetic anhydride
Home » datasheet » Boiling point of acetic anhydrideBoiling point of acetic anhydride
Boiling Point Of Acetic Anhydride. How many grams of salicylic acid are needed to make 1000 1-gram tablets of aspirin. Assume 100 percent yield Solution. Pure acetic acid often called glacial acetic acid is a corrosive colourless liquid boiling point 1179 C 2442 F. B formation of addition product with 24 DNP reagent c Silver mirror formation with Tollens reagent d existence of alpha and beta forms of glucose.
 Acetic Anhydride An Overview Sciencedirect Topics From sciencedirect.com
Acetic Anhydride An Overview Sciencedirect Topics From sciencedirect.com
Sublimes at 239F at 5 mm Hg pressure. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Acetic acid or acetic anhydride can explode with nitric acid if not kept cold. Melting point 166 C 619 F that is completely miscible with water. Addition of a small amount of water.
The objective of this experiment was to synthesize acetyl salicylic acid aspirin from salicylic acid and acetic anhydride and then determine the purity of the samples from the observed melting point temperature ranges and the efficiency of the techniques performed from the percent yield of our samples compared to the theoretical yield of 26 grams of aspirin.
The formula for this reaction is C 7 H 6 O 3 C 4 H 6 O 3 C 9 H 8 O 4 HC 2 H 3 O 2. Williamsons synthesis of preparing dimethyl ether is. Melting point 166 C 619 F that is completely miscible with water. And at 198F and 1 mm Hg pressure. B formation of addition product with 24 DNP reagent c Silver mirror formation with Tollens reagent d existence of alpha and beta forms of glucose. Assume 100 percent yield Solution.
 Source: wikiwand.com
Source: wikiwand.com
Aspirin is prepared from the reaction of salicylic acid C 7 H 6 O 3 and acetic anhydride C 4 H 6 O 3 to produce aspirin C 9 H 8 O 4 and acetic acid HC 2 H 3 O 2. Pure acetic acid often called glacial acetic acid is a corrosive colourless liquid boiling point 1179 C 2442 F. The formula for this reaction is C 7 H 6 O 3 C 4 H 6 O 3 C 9 H 8 O 4 HC 2 H 3 O 2. Commonly abbreviated Ac 2 O it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesisIt is a colorless liquid that smells strongly of acetic acid which is formed by its reaction with moisture in the air. During the production of terephthalic acid n-xylene is oxidized in the presence of acetic acid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The formula for this reaction is C 7 H 6 O 3 C 4 H 6 O 3 C 9 H 8 O 4 HC 2 H 3 O 2. The formula for this reaction is C 7 H 6 O 3 C 4 H 6 O 3 C 9 H 8 O 4 HC 2 H 3 O 2. Alcohol - ethyl grain ethanol C. How many grams of salicylic acid are needed to make 1000 1-gram tablets of aspirin. Quickly translate words and phrases between English and over 100 languages.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. The product of the condensation of two molecules of acetic acid is acetic anhydride. Aspirin an acetyl derivative of. A Formation of pentaacetate of glucose with acetic anhydride. Moderately toxic and an irritant.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Melting point 166 C 619 F that is completely miscible with water. The main process involves dehydration of acetic acid to give ketene at 700750 C. Alcohol - ethyl grain ethanol C. Sublimes at 239F at 5 mm Hg pressure. The objective of this experiment was to synthesize acetyl salicylic acid aspirin from salicylic acid and acetic anhydride and then determine the purity of the samples from the observed melting point temperature ranges and the efficiency of the techniques performed from the percent yield of our samples compared to the theoretical yield of 26 grams of aspirin.
 Source: sciencedirect.com
Source: sciencedirect.com
Moderately toxic and an irritant. Sublimes at 239F at 5 mm Hg pressure. And at 198F and 1 mm Hg pressure. A Formation of pentaacetate of glucose with acetic anhydride. The formula for this reaction is C 7 H 6 O 3 C 4 H 6 O 3 C 9 H 8 O 4 HC 2 H 3 O 2.
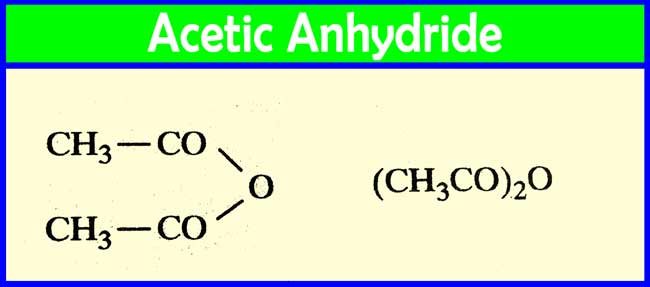 Source: chemistrypage.in
Source: chemistrypage.in
Aspirin is prepared from the reaction of salicylic acid C 7 H 6 O 3 and acetic anhydride C 4 H 6 O 3 to produce aspirin C 9 H 8 O 4 and acetic acid HC 2 H 3 O 2. How many grams of salicylic acid are needed to make 1000 1-gram tablets of aspirin. During these processes detonating mixtures may be produced. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Williamsons synthesis of preparing dimethyl ether is.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Succinic anhydride appears as colorless needles or white crystalline solid. A Formation of pentaacetate of glucose with acetic anhydride. Addition of a small amount of water. The worldwide production of acetic anhydride is a major application and uses approximately 25 to 30 of the global production of acetic acid. Acetic acid anhydride CH 3 COO 2 O.
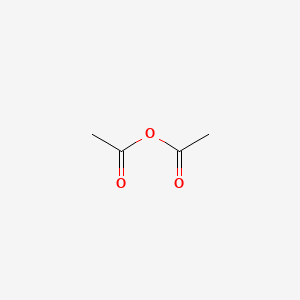
The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Aspirin is prepared from the reaction of salicylic acid C 7 H 6 O 3 and acetic anhydride C 4 H 6 O 3 to produce aspirin C 9 H 8 O 4 and acetic acid HC 2 H 3 O 2. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Williamsons synthesis of preparing dimethyl ether is. The main process involves dehydration of acetic acid to give ketene at 700750 C.
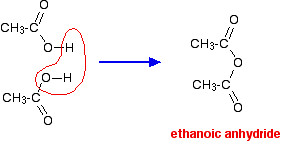 Source: chemguide.co.uk
Source: chemguide.co.uk
The objective of this experiment was to synthesize acetyl salicylic acid aspirin from salicylic acid and acetic anhydride and then determine the purity of the samples from the observed melting point temperature ranges and the efficiency of the techniques performed from the percent yield of our samples compared to the theoretical yield of 26 grams of aspirin. Pure acetic acid often called glacial acetic acid is a corrosive colourless liquid boiling point 1179 C 2442 F. The product of the condensation of two molecules of acetic acid is acetic anhydride. The formula for this reaction is C 7 H 6 O 3 C 4 H 6 O 3 C 9 H 8 O 4 HC 2 H 3 O 2. Addition of a small amount of water.
Source: chemspider.com
Acetic acid anhydride CH 3 COO 2 O. And at 198F and 1 mm Hg pressure. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. The product of the condensation of two molecules of acetic acid is acetic anhydride. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of acetic anhydride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.