Boiling point of acetamide
Home » datasheet » Boiling point of acetamideBoiling point of acetamide
Boiling Point Of Acetamide. Boiling point C K b Ckgmol Freezing point C K f Ckgmol Data source. Melting point and boiling point of nonmetals are significantly lower compared to metals Carbon being the exception. A 2-MethylButane b 2-MethylPropane c 22-Dimethylpropane d n-Pentane. Acetamide 150 o C.
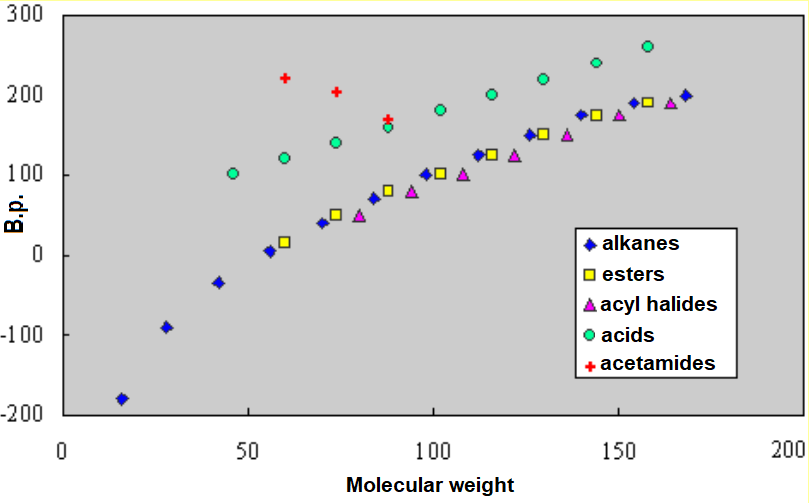 Phys Properties Of Amides From qorganica.es
Phys Properties Of Amides From qorganica.es
The melting and boiling points of covalent compounds are low. When boiling points are known purification by distillation as a means of removing impurities should be employed. 304 deg C VP exp database. Ethanoic Acid has one hydrogen bond donor and two hydrogen bond acceptor atoms. By Cepheus derivative work. The OD value in super-high-dose acetamide treatment group was significantly higher than that in the TET exposure group at 3 hr after treatment p.
1143 deg C BP exp database.
Sl H 2 O. 200E-04 mm Hg at 25 deg C Subcooled liquid VP. Which of the following will have the lowest boiling point. Sl H 2 O. For the compounds with the same molecular mass boiling point decreases with an increase in branching. Metabolite observed in cancer metabolism.
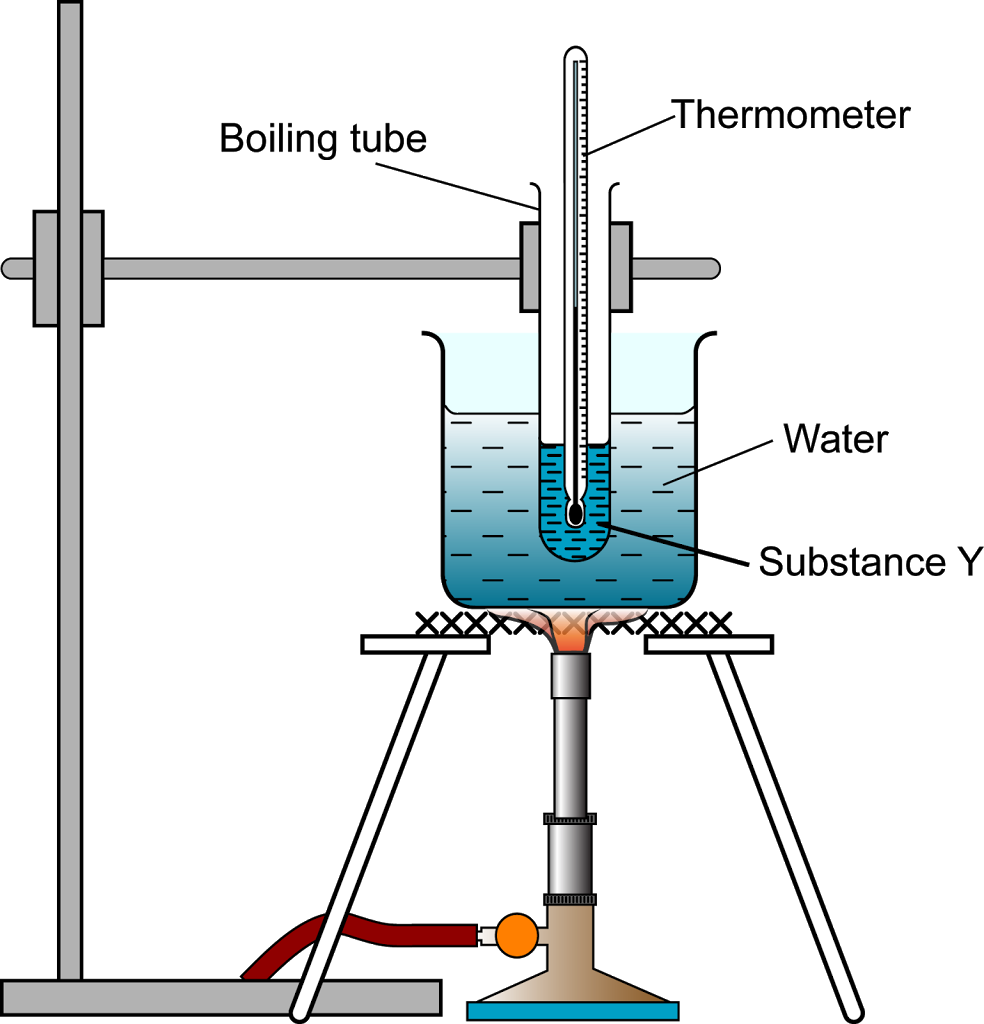 Source: spmchemistry.blog.onlinetuition.com.my
Source: spmchemistry.blog.onlinetuition.com.my
A 2-MethylButane b 2-MethylPropane c 22-Dimethylpropane d n-Pentane. 1179 307 166 390 K b K f. 1143 deg C BP exp database. The graph below shows a range of solvents with two well known HBP solvents DMF Dimethylformamide DMSO Dimethylsulfoxide occurring to the left of water which is in yellow on the graph. The melting and boiling points of covalent compounds are low.
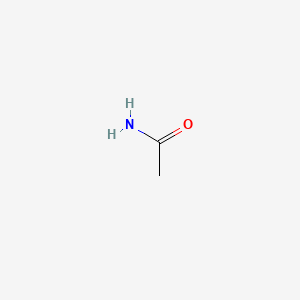
Benzene has been used as a solvent extensively reactions and purification by chromatography and crystallization but is considered a very dangerous. Formic acid systematically named methanoic acid is the simplest carboxylic acid and has the chemical formula H 2 CO 2It is an important intermediate in chemical synthesis and occurs naturally most notably in some antsThe word formic comes from the Latin word for ant formica referring to its early isolation by the distillation of ant bodies. Sl H 2 O. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. It is a member of acetamides and a monocarboxylic acid amide.
 Source: qorganica.es
Source: qorganica.es
It was significantly. Formic acid systematically named methanoic acid is the simplest carboxylic acid and has the chemical formula H 2 CO 2It is an important intermediate in chemical synthesis and occurs naturally most notably in some antsThe word formic comes from the Latin word for ant formica referring to its early isolation by the distillation of ant bodies. How much acetamide in grams was dissolved to yield the solution. 1179 307 166 390 K b K f. Which of the following will have the lowest boiling point.
 Source: in.pinterest.com
Source: in.pinterest.com
Acetamide 2-chloro-N-ethoxymethyl-N-2-ethyl-6-methylphenyl-C 14 H 20 ClNO 2. Which of the following will have the lowest boiling point. How much acetamide in grams was dissolved to yield the solution. Metabolite observed in cancer metabolism. Ethanoic Acid is completely soluble in organic solvents such as carbon tetrachloride and carbon.
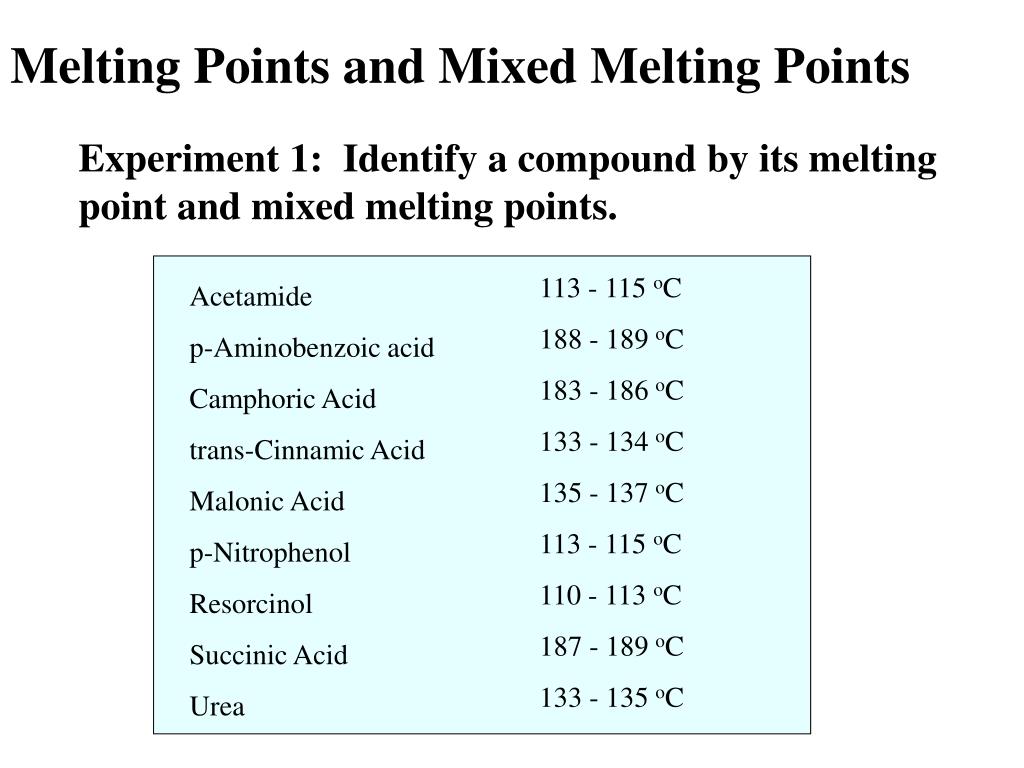 Source: slideserve.com
Source: slideserve.com
Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. Sl H 2 O. Ammonium hydroxide conc 20 o C. Acetic acid 20 o C. Boiling point increases with increase in molecular mass.
 Source: chemsynthesis.com
Source: chemsynthesis.com
C 2 H 6 N 2 O. I H 2 O eth. Boiling point C Ethanol C 2 H 5 OH-117. Which of the following will have the lowest boiling point. By Cepheus derivative work.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Melting point and boiling point of nonmetals are significantly lower compared to metals Carbon being the exception. The solubility of pure Ethanoic Acid in water is 100 mgmL at 25C. The changes in the high-dose acetamide treatment group were similar to those in the TET exposure group but became more like those in the control groups after 48 hr. Benzene has been used as a solvent extensively reactions and purification by chromatography and crystallization but is considered a very dangerous. The boiling point of Ethanoic Acid is 118C and its melting point is 16C.
 Source: chemsynthesis.com
Source: chemsynthesis.com
The boiling point is specific for the given substanceFor example the boiling point of. 1143 deg C BP exp database. It is a member of acetamides and a monocarboxylic acid amide. When boiling points are known purification by distillation as a means of removing impurities should be employed. Boiling point increases with increase in molecular mass.
 Source: guidechem.com
Source: guidechem.com
Ammonium hydroxide conc 20 o C. 1179 307 166 390 K b K f. I H 2 O eth. 0000207 Modified Grain method MP exp database. It was significantly.
 Source: pt.slideshare.net
Source: pt.slideshare.net
For the compounds with the same molecular mass boiling point decreases with an increase in branching. 462 234 1115. C 15 H 20 N 2 O 4 S. The boiling point of Ethanoic Acid is 118C and its melting point is 16C. Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each molecule are given for 150 different alcohols and acids.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of acetamide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.