Boiling point of 2 methylbutane
Home » datasheet » Boiling point of 2 methylbutaneBoiling point of 2 methylbutane
Boiling Point Of 2 Methylbutane. Point boiling point density heat of formation-138-159-1-12 0579 0549-304-324 We refer to these two different compounds having identical molecular formulas as isomers equal units. Isopentane also called methylbutane or 2-methylbutane is a branched-chain saturated hydrocarbon an alkane with five carbon atoms with formula C 5 H 12 or CHCH 3 2 C 2 H 5. Alkyl halides undergo elimination via two common mechanisms known as E2 and E1 which show some similarities to S N 2 and S N 1 respectively. Decomposes above 25 to methane Solubility.
 2 Methylbutane Anhydrous 99 78 78 4 From sigmaaldrich.com
2 Methylbutane Anhydrous 99 78 78 4 From sigmaaldrich.com
That is their skeletons are different. 0 C 32 F. Isopentane also called methylbutane or 2-methylbutane is a branched-chain saturated hydrocarbon an alkane with five carbon atoms with formula C 5 H 12 or CHCH 3 2 C 2 H 5. Decomposes above 25 to methane Solubility. When a metal atom combines with non-metal atom the non-metal atom will i gain electrons and increase in size ii gain electrons and increase in size iii lose electrons and increase in size iv lose electrons and. Point boiling point density heat of formation-138-159-1-12 0579 0549-304-324 We refer to these two different compounds having identical molecular formulas as isomers equal units.
Which of the following will have the lowest boiling point.
The yield of gasoline may be doubled by converting higher or lower boiling point fractions into hydrocarbons in the gasoline range. It is also the least dense liquid at standard conditions. Citation needed The normal boiling point is just a few. 273 K Boiling point. Isomers can be different in the connectivity of the atoms. I 2-MethylButane ii 2-MethylPropane iii 22-Dimethylpropane iv n-Pentane.

This fractional distillation process yields approximately 250 mL of straight-run gasoline for each liter of crude oil. Two of the main processes used to perform this conversion is. Point boiling point density heat of formation-138-159-1-12 0579 0549-304-324 We refer to these two different compounds having identical molecular formulas as isomers equal units. 273 K Boiling point. That is their skeletons are different.

The yield of gasoline may be doubled by converting higher or lower boiling point fractions into hydrocarbons in the gasoline range. It is also the least dense liquid at standard conditions. In E2 elimination shows a second order rate law and occurs in a single concerted step proton abstraction at C α occurring at the same time as C β-X bond cleavage. In E1 elimination goes via a first order rate law in two steps C β-X bond. 0 C 32 F.
 Source: scbt.com
Source: scbt.com
The crude oil is separated according to different boiling points into fractions. Alkyl halides undergo elimination via two common mechanisms known as E2 and E1 which show some similarities to S N 2 and S N 1 respectively. Isopentane also called methylbutane or 2-methylbutane is a branched-chain saturated hydrocarbon an alkane with five carbon atoms with formula C 5 H 12 or CHCH 3 2 C 2 H 5. Isopentane is an extremely volatile and extremely flammable liquid at room temperature and pressure. The yield of gasoline may be doubled by converting higher or lower boiling point fractions into hydrocarbons in the gasoline range.
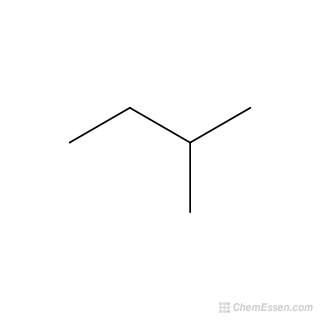 Source: molinstincts.com
Source: molinstincts.com
273 K Boiling point. Or isomers can have the same connectivity of atoms but differ in their orientation in space stereoisomers. Alkyl halides undergo elimination via two common mechanisms known as E2 and E1 which show some similarities to S N 2 and S N 1 respectively. Two of the main processes used to perform this conversion is. It is also the least dense liquid at standard conditions.
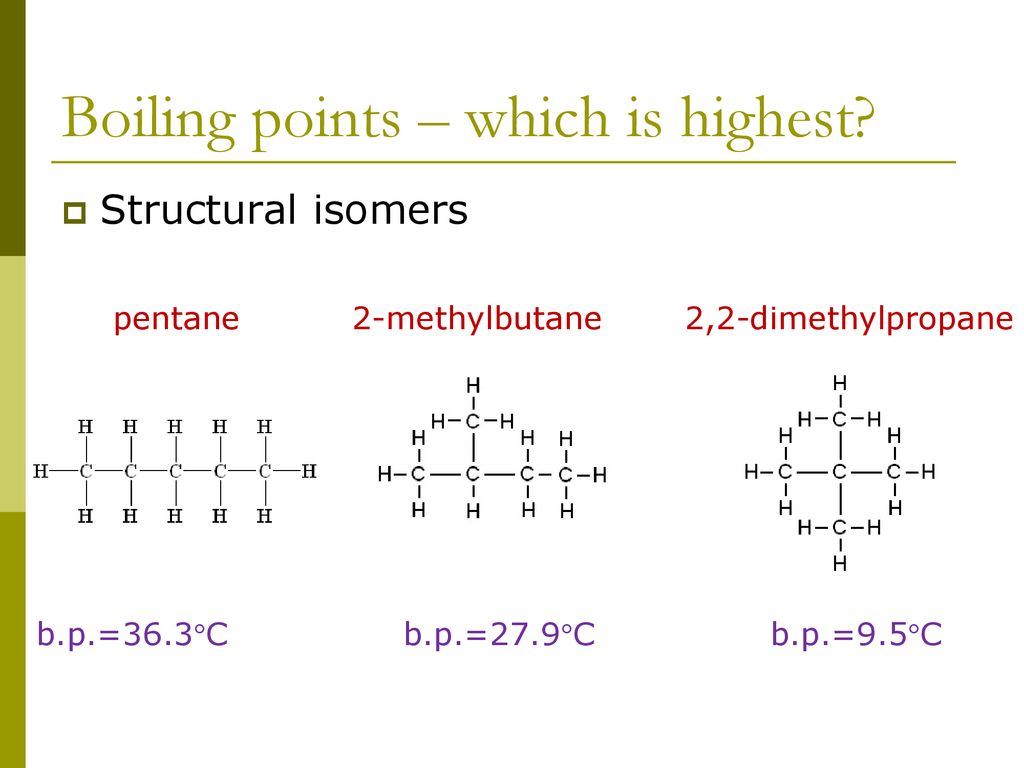 Source: slideplayer.com
Source: slideplayer.com
Which of the following will have the lowest boiling point. I 2-MethylButane ii 2-MethylPropane iii 22-Dimethylpropane iv n-Pentane. In E1 elimination goes via a first order rate law in two steps C β-X bond. Ether pentane 2-methylbutane Thermochemistry Std enthalpy of. Point boiling point density heat of formation-138-159-1-12 0579 0549-304-324 We refer to these two different compounds having identical molecular formulas as isomers equal units.
 Source: openwetware.org
Source: openwetware.org
273 K Boiling point. It is also the least dense liquid at standard conditions. The crude oil is separated according to different boiling points into fractions. Ether pentane 2-methylbutane Thermochemistry Std enthalpy of. Citation needed The normal boiling point is just a few.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
273 K Boiling point. The crude oil is separated according to different boiling points into fractions. This fractional distillation process yields approximately 250 mL of straight-run gasoline for each liter of crude oil. Citation needed The normal boiling point is just a few. The yield of gasoline may be doubled by converting higher or lower boiling point fractions into hydrocarbons in the gasoline range.

It is also the least dense liquid at standard conditions. When a metal atom combines with non-metal atom the non-metal atom will i gain electrons and increase in size ii gain electrons and increase in size iii lose electrons and increase in size iv lose electrons and. Isomers can be different in the connectivity of the atoms. Or isomers can have the same connectivity of atoms but differ in their orientation in space stereoisomers. In E1 elimination goes via a first order rate law in two steps C β-X bond.
 Source: youtube.com
Source: youtube.com
Ether pentane 2-methylbutane Thermochemistry Std enthalpy of. Which of the following will have the lowest boiling point. Ether pentane 2-methylbutane Thermochemistry Std enthalpy of. This fractional distillation process yields approximately 250 mL of straight-run gasoline for each liter of crude oil. In E2 elimination shows a second order rate law and occurs in a single concerted step proton abstraction at C α occurring at the same time as C β-X bond cleavage.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Two of the main processes used to perform this conversion is. Two of the main processes used to perform this conversion is. Or isomers can have the same connectivity of atoms but differ in their orientation in space stereoisomers. In E2 elimination shows a second order rate law and occurs in a single concerted step proton abstraction at C α occurring at the same time as C β-X bond cleavage. Citation needed The normal boiling point is just a few.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point of 2 methylbutane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.