Boiling point of 2 2 dimethylpropane
Home » datasheet » Boiling point of 2 2 dimethylpropaneBoiling point of 2 2 dimethylpropane
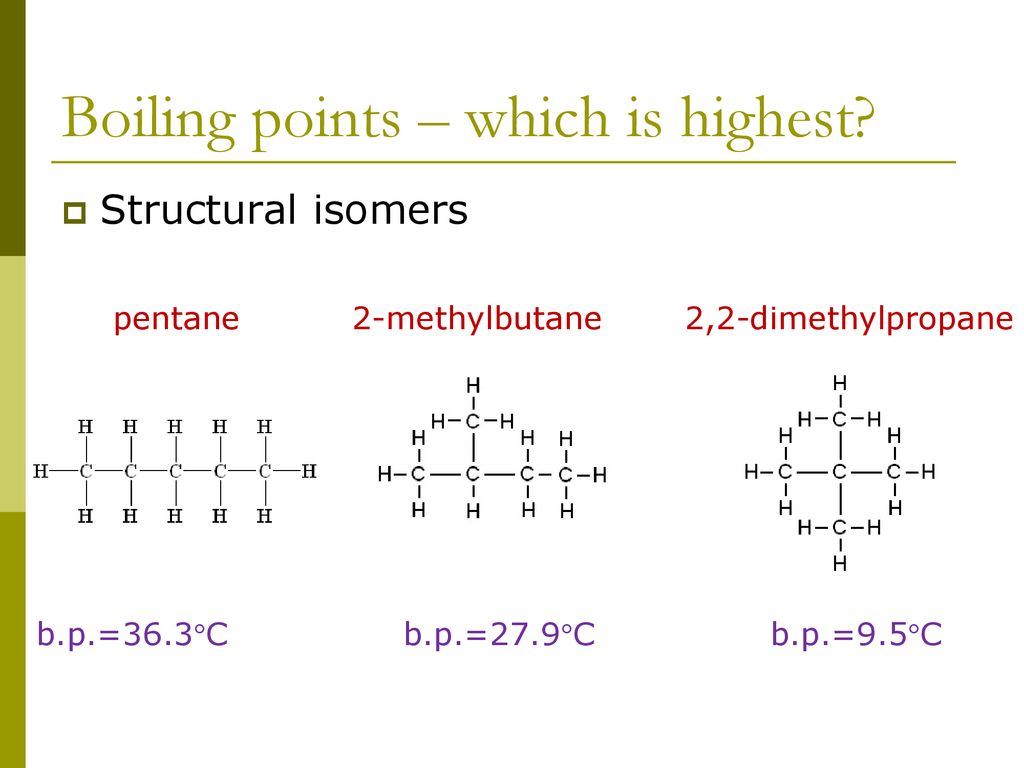
Boiling Point Of 2 2 Dimethylpropane. The yield of gasoline may be doubled by converting higher or lower boiling point fractions into hydrocarbons in the gasoline range. That is their skeletons are different. This reaction slows down and stops. For example 22-dimethylpropane neopentane has a lower boiling point than pentane.
 Chemistry Organic Molecules Trends In Boiling Temp Viscosity And Flash Point From dynamicscience.com.au
Chemistry Organic Molecules Trends In Boiling Temp Viscosity And Flash Point From dynamicscience.com.au
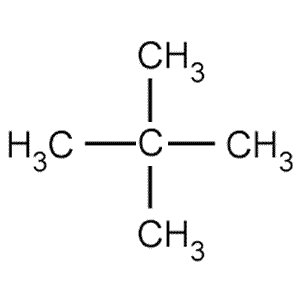
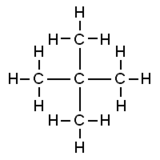
Neopentane also called 22-dimethylpropane is a double-branched-chain alkane with five carbon atoms. C has the highest vapour. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. 1 Pentana C 5 H 12. For example 22-dimethylpropane neopentane has a low boiling point than pentane. Therefore it has less surface area than the more cylindrical pentane molecule.
Email protected 2011-01-01.
Its a force of attraction that affects all molecules. Interestingly the pattern is not observed for the melting points. For example 22-dimethylpropane neopentane has a lower boiling point than pentane. Point boiling point density heat of formation-138-159-1-12 0579 0549-304-324 We refer to these two different compounds having identical molecular formulas as isomers equal units. As a result the van der Waals forces are smaller in neopentane and the boiling point is low. C5h11cl isomers - egzotyka-lastminutepl.
Source: chemspider.com
The melting point of. Two of the main processes used to perform this conversion is. The crude oil is separated according to different boiling points into fractions. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. Intermolecular Forces in Liquids and Gases.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Which of the following will have the lowest boiling point. D more than one correct response E no correct response. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. Neopentane is more spherical than pentane. A strong signal at 1700.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Flash point is an important parameter for safety considerations especially during storage and transportation of volatile petroleum products ie LPG light naphtha gasoline in a high-temperature environment. The IMFs in propanone are dispersion and dipole-dipole. 1 Pentana C 5 H 12. Apr 17 2019 Model 1. Generally flash point increases with an increase in boiling point.
 Source: slideplayer.com
Source: slideplayer.com
BOILING POINT C A. 31 Define the term. Since the intermolecular attractive forces differ in the two substances the enthalpy of vaporization will differ. Which of the following will have the lowest boiling point. Boiling point increases with increase in molecular mass.
Source: quora.com
And given Sr 1041 K Ba 1002 K and Ra 973 K perhaps the question might be why is calciums melting point a tad higher than you might expect. Answer d n-Pentane Explanation. The boiling point of a liquid varies depending upon the surrounding environmental pressure. The surrounding temperature around a storage tank should always. Which of the following will have the lowest boiling point.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Remember for the boiling point it increased with less branching because of the increased surface. Which of the following statements concerning the boiling points of specific alkanes is correct. C has the highest vapour. Email protected 2011-01-01. In shape neopentane is more spherical than that of pentane.
Source: synquestlabs.com
CeCCH_3_4 - Two branches-22-Dimethylpropane Neo-pentane mathrmbp2825mathrmK This is due to the fact that branching of the chain makes the molecule more compact and thereby decreases the surface area. For the compounds with the same molecular mass boiling point decreases with an increase in branching. The molecular mass of 2-Methylbutane. Boiling point increases with increase in molecular mass. 1 33 Explain the trend in the boiling points from compound.
Index of refraction 14. Therefore it has less surface area than the more cylindrical pentane molecule. As a result the boiling point will be higher for propanoic acid than for hex-1-ene. A liquid at high pressure. Its a force of attraction that affects all molecules.
Source: en.wikipedia.org
1-Bromo-22-dimethylpropane C5H11Br CID 12415 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. 1 Pentana C 5 H 12. Neopentane is more spherical than pentane. Reactivity Series in chemistry is an experimental structural and logical progression of series of metals in order of reactivity from highest to lowest. Which of the following will have the lowest boiling point.
 Source: store.apolloscientific.co.uk
Source: store.apolloscientific.co.uk
Isomers can be different in the connectivity of the atoms. Which of the following will have the lowest boiling point. Remember for the boiling point it increased with less branching because of the increased surface. Occurrence Natural gas - principally methane CH 4 Petroleum oil - mixture up to ca. Index of refraction 14.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of 2 2 dimethylpropane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.