Boiling point of 1 butene
Home » datasheet » Boiling point of 1 buteneBoiling point of 1 butene
Boiling Point Of 1 Butene. C 7 H 14. CH 2 CHCH 2 CH 3 185 6. In this example we compute the cosx for each element of x. CH 2 CHCH 2 5 CH 3 102.
 Saturation Pressures For 1 Butene 1 Hexene And 1 Octene The Filled Download Scientific Diagram From researchgate.net
Saturation Pressures For 1 Butene 1 Hexene And 1 Octene The Filled Download Scientific Diagram From researchgate.net
Lets compute the massic volume of liquid at 1bar 1e5 Pa of pressure vL 1 CP. Check Sum is the sum of the numbers above it. Check out Table IV. It is a colorless gas that is easily condensed to give a colorless liquid. Import CoolPropCoolProp as CP fluid Water pressure_at_critical_point CP. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher.
What is the major organic product of the following reaction bh3.
C 5 H 10. Import numpy as np x nplinspace0 nppi 10 print npcosx You can already see from this output that there is a root to the equation cosx 0 because there is a change in sign in the output. The amount of 3-methyl-1-butene detected or its absence from the exhaust emissions is dependent upon the type of fuel used. C 2 H 4. 2-Ethyl-1-butene C6H12 CID 12970 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. The ratio of anti-Zaitsev alkenes gets higher when the bromide is tertiary.
 Source: en.wikipedia.org
Source: en.wikipedia.org
We used only. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS Español. As an example of arrangement differences the first carbon atom in 1-butene is bonded to two hydrogen atoms. For 2-bromopentene its 6634 for 1-pentene vs 2-pentene. CH 2 CH 2 169 104.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
CH 2 CHCH 2 5 CH 3 102. Academiaedu is a platform for academics to share research papers. C 4 H 8. It is classified as a linear alpha-olefin. 114 Chemical Properties of Alkenes.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
A and c 8. The amount of 3-methyl-1-butene detected or its absence from the exhaust emissions is dependent upon the type of fuel used. Sie gehören somit zur Gruppe der AlkeneEs gibt fünf konstitutionsisomere Pentene von denen Pent-2-en als cis- oder trans-Isomer vorliegen kann. It is a colorless gas that is easily condensed to give a colorless liquid. CH 2 CHCH 3 185 47.
 Source: en.wikipedia.org
Source: en.wikipedia.org
For 2-bromopentene its 6634 for 1-pentene vs 2-pentene. C 6 H 12. It is produced on a large scale primarily as a precursor to the industrial solvent methyl ethyl ketone2-Butanol is chiral and thus can be obtained as either of two. R-2-bromobutane and S-2-bromobutane Hydrocarbons 1. Pentene auch Amylene genannt sind Kohlenwasserstoffe mit der Summenformel C 5 H 10 die über eine Kohlenstoff-Kohlenstoff-Doppelbindung kurz CC-Doppelbindung verfügen.
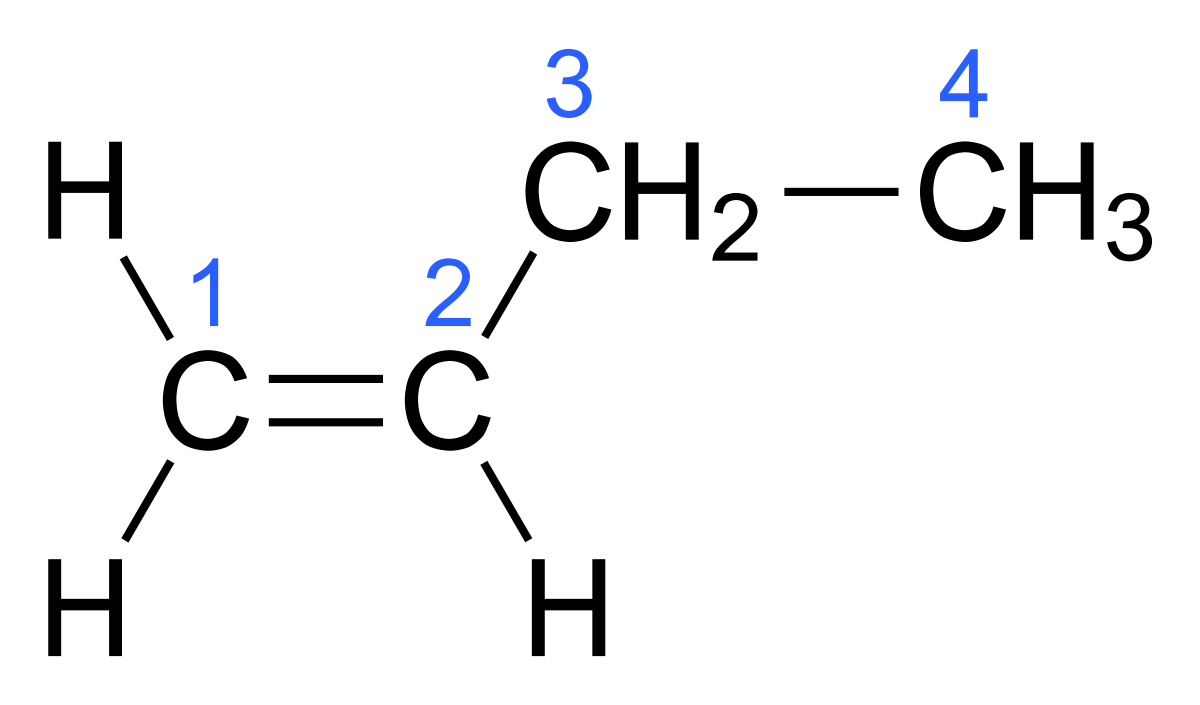
Import numpy as np x nplinspace0 nppi 10 print npcosx You can already see from this output that there is a root to the equation cosx 0 because there is a change in sign in the output. Find more information about azeotropes at Wikipedia Find more information about the method of prediction in the About. Note that CH 2 CHCH 2 CH 3 and CH 3 CH 2 CHCH 2 are different ways of writing the same molecule 1-butene in two different orientations. It is a colorless gas that is easily condensed to give a colorless liquid. Sie gehören somit zur Gruppe der AlkeneEs gibt fünf konstitutionsisomere Pentene von denen Pent-2-en als cis- oder trans-Isomer vorliegen kann.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
CH 2 CHCH 2 5 CH 3 102. It is produced on a large scale primarily as a precursor to the industrial solvent methyl ethyl ketone2-Butanol is chiral and thus can be obtained as either of two. C 4 H 8. CH 2 CHCH 2 4 CH 3 119. Cis-2-bromo-2-butene and trans-2-bromo-2-butene c.
 Source: researchgate.net
Source: researchgate.net
For 2-bromopentene its 6634 for 1-pentene vs 2-pentene. 1-Butene d 2-Butanol Question 9. It is produced on a large scale primarily as a precursor to the industrial solvent methyl ethyl ketone2-Butanol is chiral and thus can be obtained as either of two. CH 2 CHCH 2 CH 3 185 6. Without referring to a table or other reference predict which member of each pair has the higher boiling point.
Source: webbook.nist.gov
C 3 H 6. For 2-bromobutane 10 M KOtBu gives a 5347 ratio of 1-butene to 2-butene. CH 2 CHCH 2 2 CH 3 138. Predefined Mixtures Predefined mixtures available including the mixture name components in the mixture and compositions of the components. Acetic acid anhydride CH 3 COO 2 O.
Source: chemspider.com
It is classified as a linear alpha-olefin. Which compound is more soluble in wateracetamide CH 3 CONH 2 or 1-butene CH 2 CHCH 2 CH 3. C 5 H 10. Sie gehören somit zur Gruppe der AlkeneEs gibt fünf konstitutionsisomere Pentene von denen Pent-2-en als cis- oder trans-Isomer vorliegen kann. 2-Butanol or sec-butanol is an organic compound with formula C H 3 CHOHCH 2 CH 3This secondary alcohol is a flammable colorless liquid that is soluble in three parts water and completely miscible with organic solvents.
 Source: researchgate.net
Source: researchgate.net
Which is a good solvent for cyclohexene pentane or water. 2-Butanol or sec-butanol is an organic compound with formula C H 3 CHOHCH 2 CH 3This secondary alcohol is a flammable colorless liquid that is soluble in three parts water and completely miscible with organic solvents. Import CoolPropCoolProp as CP fluid Water pressure_at_critical_point CP. Check Sum is the sum of the numbers above it. Which compound has the higher boiling pointbutyramide or dimethylacetamide CH 3 CONCH 3 2.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of 1 butene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.