Boiling point of 1 butanol
Home » datasheet » Boiling point of 1 butanolBoiling point of 1 butanol
Boiling Point Of 1 Butanol. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. A strong signal at 1700. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. FIRST AID MEASURES Eye Contact.
 Observed And Literature Values Of Boiling Point Density And Viscosity Download Table From researchgate.net
Observed And Literature Values Of Boiling Point Density And Viscosity Download Table From researchgate.net
Analytical 4 ACS reagent 2 BioReagent 2 Technique. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. E Molecular Structure and Spectra 1. Whether fusel alcohol contributes to hangover symptoms is a matter of scientific debate.
Fusel alcohols or fuselol also sometimes called fusel oils in Europe are mixtures of several higher alcohols those with more than 2 carbons chiefly amyl alcohol produced as a by-product of alcoholic fermentation.
Boiling Point C Feature. FIRST AID MEASURES Eye Contact. This lower-boiling ternary azeotrope is removed preferentially leading to water-free ethanol. The values in the table below except as noted have been extracted from online and hardbound compilations. The boiling point of pentane C 5 H 12 MW 72 is 36 C 97 F close to the. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation.
Source: chemspider.com
Determine the double bond stereochemistry E or Z for the following molecules. Analytical 4 ACS reagent 2 BioReagent 2 Technique. However the boiling point decreases quite significantly as we move towards the more branched isomers. This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol.
 Source: ddbst.com
Source: ddbst.com
Boiling point and melting point d. Symmetry and Melting Point. However gas molecules are not point masses and there are many cases gases need to be treated as non-idealJohannes D. This lower-boiling ternary azeotrope is removed preferentially leading to water-free ethanol. Analytical 4 ACS reagent 2 BioReagent 2 Technique.
 Source: thermopedia.com
Source: thermopedia.com
The ideal gas law treats the molecules of a gas as point particles with perfectly elastic collisions. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. Determine the double bond stereochemistry E or Z for the following molecules. The word Fusel is German for bad liquor. Boiling point and melting point d.
 Source: iea-amf.org
Source: iea-amf.org
Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. Toxicological Data on Ingredients. Remember the order of increasing intramolecular interactions in covalent compounds. In fact the boiling points of ethers are much closer to those of alkanes with similar molecular weights. Symmetry and Melting Point.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. A Japanese study in 2003 concluded that the. The boiling point of pentane C 5 H 12 MW 72 is 36 C 97 F close to the. The values in the table below except as noted have been extracted from online and hardbound compilations. Van der Waals suggested a modification to take into account molecular size and molecular interaction forces.
Source: carlroth.com
This mixture is an azeotrope with a boiling point of 781 C 1726 F and cannot be further purified by distillation. A strong signal at 1700. Analytical 4 ACS reagent 2 BioReagent 2 Technique. Kemme and Kreps 1969. Determine the double bond stereochemistry E or Z for the following molecules.
Index of refraction 14. Potassium tert-butoxide being an ionic compound has the highest melting point. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Index of refraction 14. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights.
 Source: researchgate.net
Source: researchgate.net
The blood concentration of 3-methyl-1-butanol decreased from 37 mg100 ml at 1 hr ie 15 min after the last pentanol injection to 1-butanol and the other primary pentanols are conjugated to yield the glucuronide and 7-10 of the administered dose is excreted as the urinary glucuronide. Symmetry and Melting Point. However the boiling point decreases quite significantly as we move towards the more branched isomers. Addition of an entraining agent such as benzene cyclohexane or heptane allows a new ternary azeotrope comprising the ethanol water and the entraining agent to be formed. Get medical attention immediately.
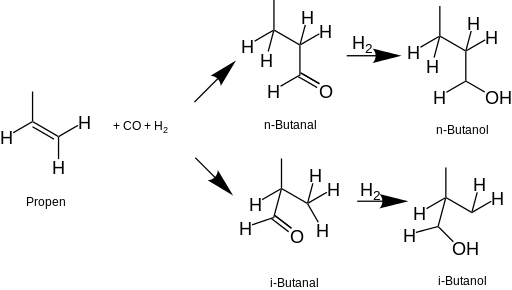 Source: en.wikipedia.org
Source: en.wikipedia.org
Aside from the. This lower-boiling ternary azeotrope is removed preferentially leading to water-free ethanol. 1-butanol is the second because of the OH group and thus hydrogen bonding. A strong signal at 1700. Immediately flush eyes with running water for at least 15 minutes keeping eyelids open.
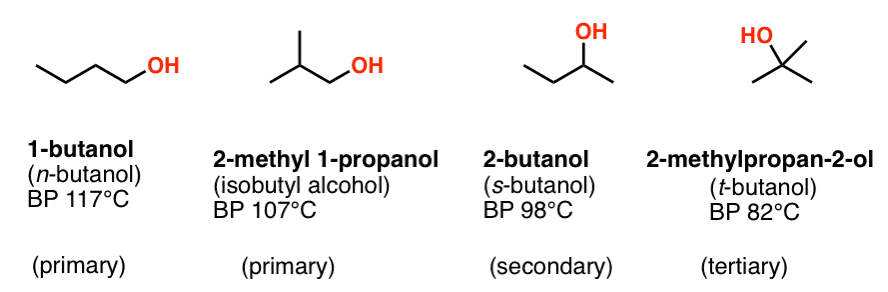 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. For example the boiling point of diethyl ether C 4 H 10 O molecular weight MW 74 is 35 C 95 F but the boiling point of 1-butanol or n-butyl alcohol. Boiling point and melting point d. Hessel and Geiseler 1965. Immediately flush eyes with running water for at least 15 minutes keeping eyelids open.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point of 1 butanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.