Boiling point hydrogen fluoride
Home » datasheet » Boiling point hydrogen fluorideBoiling point hydrogen fluoride
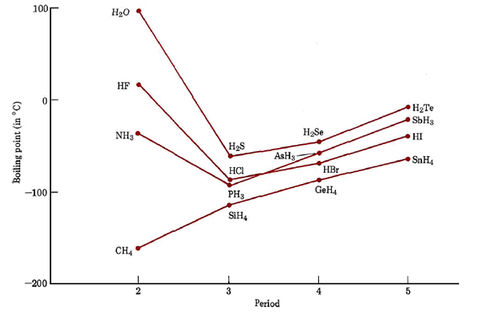
Boiling Point Hydrogen Fluoride. In a group of ammonia molecules there are not enough lone pairs to go around to satisfy all the hydrogens. Vapors are heavier than air. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Melting point - the temperature at which a solid turns into a liquid.
 Why Does Hydrogen Fluoride Have A Boiling Point So Much Lower Than That Of Water Chemistry Stack Exchange From chemistry.stackexchange.com
Why Does Hydrogen Fluoride Have A Boiling Point So Much Lower Than That Of Water Chemistry Stack Exchange From chemistry.stackexchange.com
The ICSC project is a common undertaking between the World Health Organization WHO and. HF is widely used in the petrochemical. Sodium Fluoride is an inorganic salt of fluoride used topically or in municipal water fluoridation systems to prevent dental caries. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. The hydrogen halides are colourless gases at standard conditions for temperature and pressure STP except for hydrogen fluoride which boils at 19 C. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid.
Physical Properties of Sodium fluoride NaF. Hydrogen bonding is a special type of dipole-dipole interaction that occurs between the lone pair of a highly electronegative atom typically N O or F and the hydrogen atom in a NH OH or FH bond. The stated ionization constant of hydrofluoric acid 10-315 does not reflect the true acidity of concentrated HF solutions. In the case of ammonia the amount of hydrogen bonding is limited by the fact that each nitrogen only has one lone pair. Hydrogen Water Side Effects. Boiling point - the temperature at which a liquid turns into a gas.

Ammonia has a higher boiling. Shipped as a liquid confined under its own vapor pressure. Water decomposes into a mixture of hydrogen and oxygen when an electric current is passed through the liquid. Hydrogen fluoride is a chemical compound with the chemical formula H FThis colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acidIt is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers eg. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
 Source: qsstudy.com
Source: qsstudy.com
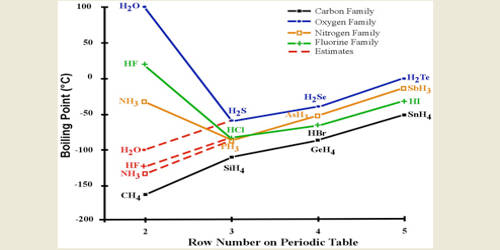
HF is widely used in the petrochemical. In water hydrogen bonding causes linkages in the water molecules which result in the boiling point of water is more than that of the other compounds. White crystals or powder. In water two. 1 mm Hg at 1971 F.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
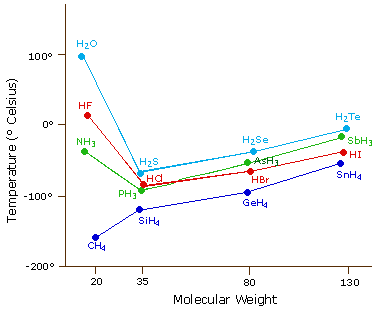
This substance is used to make many everyday products including aluminum plastics refrigerants and high octane gasoline. Hydrogen bonding also accounts for the higher boiling point of HF compared to other hydrogen halides. Hydrogen fluoride is a colorless fuming liquid below 67F 194C or a colorless gasWhen hydrogen fluoride is combined with water it is known as hydrofluoric acid a colorless liquid which in low concentrations is visually indistinguishable from water. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Anhydrous hydrogen fluoride Aqueous hydrogen fluoride HF-A Hydrofluoric acid Colorless gas or fuming liquid below 67F with a strong irritating odor.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
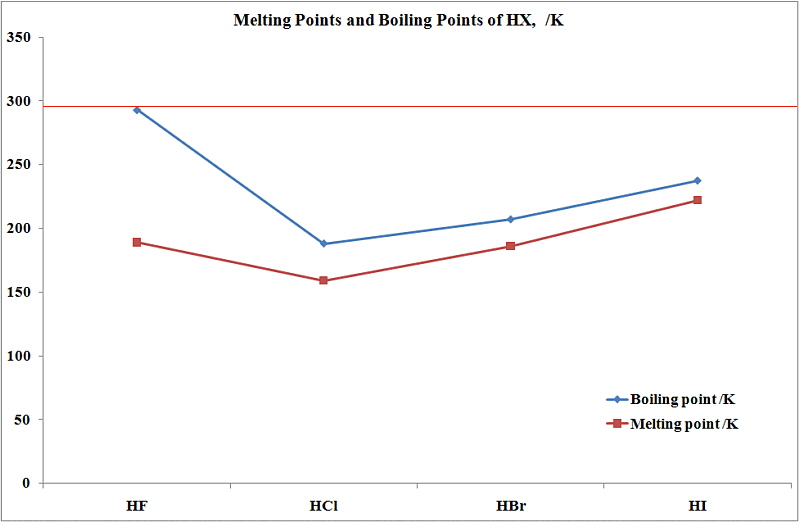
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Any substance that contains only one kind of an atom is known as an elementBecause atoms cannot be created or destroyed in a chemical reaction elements such as phosphorus P 4 or sulfur S 8 cannot be broken down into simpler substances by these reactions. The main target users are workers and those responsible for occupational safety and health. Shipped as a liquid confined under its own vapor pressure. Many have focused on water purification to solve these issues.
 Source: en.wikipedia.org
Source: en.wikipedia.org
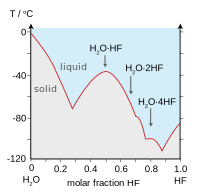
Hydrogen fluoride is a colorless fuming liquid below 67F 194C or a colorless gasWhen hydrogen fluoride is combined with water it is known as hydrofluoric acid a colorless liquid which in low concentrations is visually indistinguishable from water. Fluoride appears to bind to calcium ions in the hydroxyapatite of surface tooth enamel preventing corrosion of tooth enamel by acids. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Shipped as a liquid confined under its own vapor pressure. Physical Properties of Sodium fluoride NaF.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Straight chain compounds have higher boiling point then branched chain because in straight chain molecules are strongly entangled with each other like noodlesand have more contact with other molecules so strong force is required to remove such molecules consequently straight chain compounds have higher boiling point than branched compounds. Hydrogen fluoride is a chemical compound with the chemical formula H FThis colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acidIt is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers eg. Hydrogen fluoride is a colorless fuming liquid below 67F 194C or a colorless gasWhen hydrogen fluoride is combined with water it is known as hydrofluoric acid a colorless liquid which in low concentrations is visually indistinguishable from water. Ammonia has a higher boiling. Chemical Properties of Sodium fluoride NaF.
 Source: youtube.com
Source: youtube.com
This is why the boiling point of water is higher than that of ammonia or hydrogen fluoride. Hydrogen fluoride is a chemical compound with the chemical formula H FThis colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acidIt is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers eg. The main target users are workers and those responsible for occupational safety and health. Sodium Fluoride Structure NaF. The primary aim of the cards is to promote the safe use of chemicals in the workplace.

Corrosive to metals and tissue. White crystals or powder. Hydrogen Water Side Effects. Boiling point - the temperature at which a liquid turns into a gas. In a group of ammonia molecules there are not enough lone pairs to go around to satisfy all the hydrogens.
 Source: chem.libretexts.org
Source: chem.libretexts.org
In hydrogen fluoride the problem is a shortage of hydrogens. Hydrogen bonding is a special type of dipole-dipole interaction that occurs between the lone pair of a highly electronegative atom typically N O or F and the hydrogen atom in a NH OH or FH bond. Hydrogen fluoride anhydrous appears as a colorless fuming liquid boiling at 67F. Melting point - the temperature at which a solid turns into a liquid. Alone of the hydrogen halides hydrogen fluoride exhibits hydrogen bonding between molecules and therefore.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Ammonia has a higher boiling. Hydrogen bonding also accounts for the higher boiling point of HF compared to other hydrogen halides. This bond is much stronger than a regular hydrogen bond and can be seen in these acids when they are kept at high pressure. Shipped as a liquid confined under its own vapor pressure. The stated ionization constant of hydrofluoric acid 10-315 does not reflect the true acidity of concentrated HF solutions.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point hydrogen fluoride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.