Boiling point glycerol
Home » datasheet » Boiling point glycerolBoiling point glycerol
Boiling Point Glycerol. This decrease will affect the time it takes to cook anything in water to the extent that any food that requires five minutes to prepare at. The preparation and use of nitric acid were known to the early alchemists. CRC Handbook of Chemistry and Physics 86TH Edition. 290 C 554 F.
2 From
Make a solution of 15 glycerol. The boiling point temperature will be lower if the atmospheric pressure is decreased. Oils are triglycerides that are liquid at room temperature. Do not allow to boil dry. CRC Handbook of Chemistry and Physics 86TH Edition. Place the basin on the trivetsaucer and add boiling water to come 13 of the way up the basin.
Interactive 3D image of a saturated triacylglycerol BioTopics Saturated vs mono-unsaturated fatty acid BioTopics In.
As a result of the network of hydrogen bonding present between water molecules a high input of energy is required to transform one gram of liquid water into water vapor an energy requirement called the heat of vaporization. Uses advised against Food drug pesticide or biocidal product use. This also makes it useful as a humectant in cosmetics and food retaining water and preventing the substance from drying out. Fats are more common in animals while. Hygroscopic liquid with a high boiling point. Otherwise the triglyceride is called a mixed triglyceride.
Source:
Steam with the film lid on for 30 minutes topping up with boiling water as necessary. Chemically glycerine is a trihydric alcohol capable of being reacted as an alcohol yet stable under most conditions. The formula of glycerol is C 3 H 8 O 3. The Merck Index - An Encyclopedia of Chemicals Drugs and Biologicals. Oils are triglycerides that are liquid at room temperature.
Source:
Explosion Hazards in. Explosion Hazards in. Fire Hazards in Presence of Various Substances. Nitric acid HNO 3 colourless fuming and highly corrosive liquid freezing point 42 C 44 F boiling point 83 C 181 F that is a common laboratory reagent and an important industrial chemical for the manufacture of fertilizers and explosivesIt is toxic and can cause severe burns. 25 F -4 C Vodka 40 alcohol by volume.
 Source: sciencedirect.com
Source: sciencedirect.com
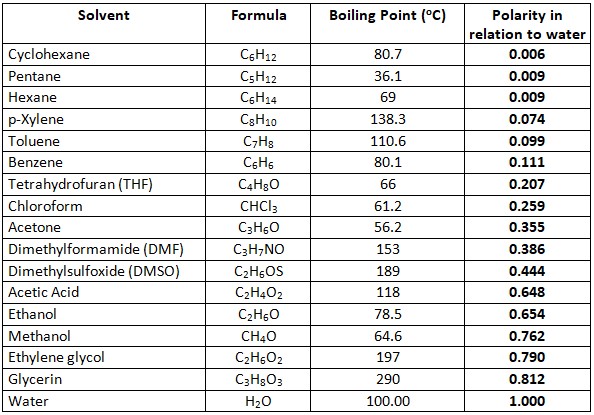
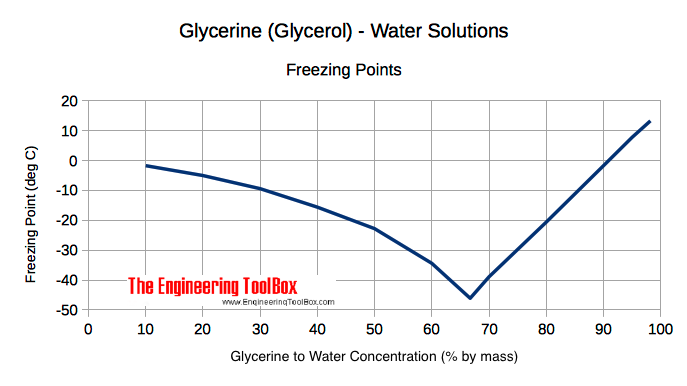
I would then do this. Make a solution of 15 glycerol. Glycerine to Water Concentration by mass weight Specific Gravity at 60 o F. Increasing the glycerine concentration above 667 will increase the freezing point as indicated below. Hexagonal anhydrous monoclinic dihydrate Octahedral hexahydrate Hazards MSDS.
 Source: researchgate.net
Source: researchgate.net
In the laboratory glycerol is a common component of solvents for. May be combustible at high temperature. Hazardous Substances Data Bank HSDB Very soluble in chloroform. 290 degree Celsius melting point. H 4 N 2.
 Source: igtpan.com
Source: igtpan.com
Boiling point of ethanol is 1733 F 785 C 1 Melting Point. The normal freezing point and the corresponding freezing point depression is tabulated below. May be combustible at high temperature. I would then do this. Trigylcerides have a glycerol backbone bonded to three fatty acids.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
436 g100 mL 0 C 45 g100 mL 7 C 529 g100 mL 20 C 105 g100 mL 96 C Solubility. Water has a heat of vaporization value of 4065 kJmol. Product Name Glycerol Cat No. Its boiling point is 290C but it also decomposes at that temperature. Trigylcerides have a glycerol backbone bonded to three fatty acids.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Vapour pressure is determined by the kinetic energy of a molecule. Place in a steamer over boiling water or alternatively use a large saucepan with a trivet or an upturned heat-resistant saucer. Normal Freezing Point o C. 1049 C Solubility in water. The formula of glycerol is C 3 H 8 O 3.
Source:
290 C 554 F. The boiling point temperature will be lower if the atmospheric pressure is decreased. This also makes it useful as a humectant in cosmetics and food retaining water and preventing the substance from drying out. CRC Handbook of Chemistry and Physics 86TH Edition. Merck and Co Inc 2001 p.
 Source: researchgate.net
Source: researchgate.net
Hygroscopic liquid with a high boiling point. Freezing Point Depression Kb o C m-1. Place in a steamer over boiling water or alternatively use a large saucepan with a trivet or an upturned heat-resistant saucer. 92094 gmol relative density. Explosion Hazards in.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Normal Freezing Point o C. Hexagonal anhydrous monoclinic dihydrate Octahedral hexahydrate Hazards MSDS. Chemically glycerine is a trihydric alcohol capable of being reacted as an alcohol yet stable under most conditions. Nitric acid HNO 3 colourless fuming and highly corrosive liquid freezing point 42 C 44 F boiling point 83 C 181 F that is a common laboratory reagent and an important industrial chemical for the manufacture of fertilizers and explosivesIt is toxic and can cause severe burns. Melting point of ethanol is -1734 F -1141 C 1.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point glycerol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.