Boiling point f argon
Home » datasheet » Boiling point f argonBoiling point f argon
Boiling Point F Argon. By contrast pressure is omitted since gaseous viscosity depends only weakly on it. Melting Point ºC Boiling Point ºC Density gcm 3 at 293 K 1. P Density g cm 3 0000825. -1958 C -3204 F Boiling point of liquid helium.
 Argon Element Information Properties And Uses Periodic Table From rsc.org
Argon Element Information Properties And Uses Periodic Table From rsc.org
Examples of uses of distillation include purification of alcohol desalination crude oil refining and making liquefied gases from air. As can be seen the boiling point of a liquid varies depending upon the surrounding environmental pressure. The temperatures corresponding to each data point are stated explicitly. Chemical name Argon Suppliers details. Not applicable GAS DENSITY. The simple structure of noble gas molecules makes them amenable to accurate theoretical treatment.
The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F.
This decrease will affect the time it takes to cook anything in water to the extent that any food that requires five minutes to prepare at. The gas cannot be liquefied by pressure above a temperature of 1223 C 1881 F and at this point a pressure of at least 48 atmospheres is required to make it liquefy. The boiling point of n butane is -04C 313F vs the boiling point of isobutane at -1175C 1085F. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition. It is in group 18 of the periodic table and is a noble gas. Humans have been using distillation since at least 3000 BC in the Indus valley.
 Source:
Source:
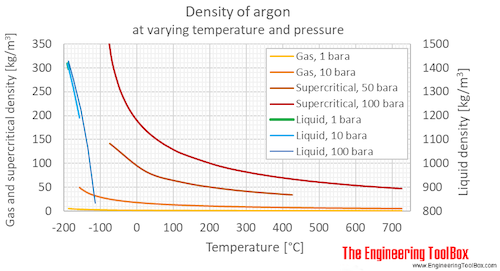
Dichlorodifluoromethane Properties - Properties of saturated liquid Dichlorodifluoromethane R-12 - CCl 2 F 2 - density specific heat capacity kinematic viscosity thermal conductivity and Prandtl number. 3732 K Boiling point of ethanol. Not applicable GAS DENSITY. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. Chemical name Argon Suppliers details.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
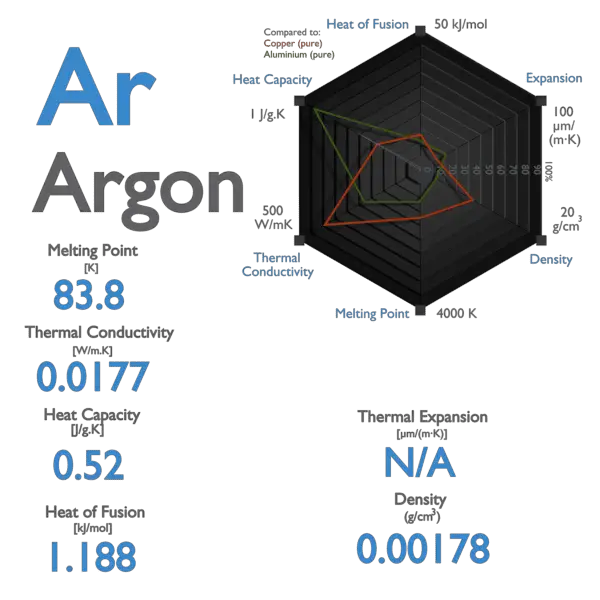
Argon Product use SyntheticAnalytical chemistry. Argon is a chemical element with the symbol Ar and atomic number 18. 8381 K 18934 C 30881 F Boiling point. To fill the gap he needed to find the second. Argon gas condenses to a colourless liquid at 1858 C 3024 F and to a crystalline solid at 1894 C 3089 F.
 Source: rsc.org
Source: rsc.org
-269 C -452 F. 13870F 211 0c and 1 atm. 3732 K Boiling point of ethanol. Have a look at this table with the elements of the periodic table arranged in order of increasing boiling points. The simple structure of noble gas molecules makes them amenable to accurate theoretical treatment.

Argon Product use SyntheticAnalytical chemistry. So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F. Boiling Points for common Liquids and Gases - Boiling temperatures for some common liquids and gases - acetone butane propane. MATERIAL SAFETY DATA SHEET - ARGON- COMPRESSED ROC Group of Companies Page 4 of 6 LOWER. The flash point is the lowest temperature at which the gas will ignite with an ignition source not to be confused with the autoignition temperature spontaneous ignition.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is in group 18 of the periodic table and is a noble gas. 56 C 1328 F Boiling point of alcohol. Not applicable GAS DENSITY. 8381 K 18934 C 30881 F Boiling point. The temperatures corresponding to each data point are stated explicitly.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
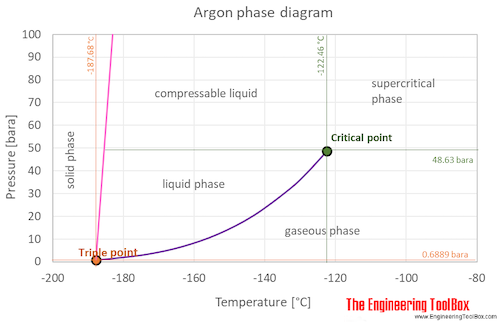
At the critical point there is no change of state when pressure is increased or if heat is added. It is in group 18 of the periodic table and is a noble gas. At the critical point there is no change of state when pressure is increased or if heat is added. 7837 C 1731 F Boiling point of nitrogen. This decrease will affect the time it takes to cook anything in water to the extent that any food that requires five minutes to prepare at.
 Source: britannica.com
Source: britannica.com
The temperatures corresponding to each data point are stated explicitly. The temperatures corresponding to each data point are stated explicitly. 100 C 212 F Boiling point of water in Kelvin. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure.
 Source: britannica.com
Source: britannica.com
56 C 1328 F Boiling point of alcohol. 7837 C 1731 F Boiling point of nitrogen. It is more than twice as abundant as. Boiling Points for common Liquids and Gases - Boiling temperatures for some common liquids and gases - acetone butane propane. 7837 C 1731 F Boiling point of methanol.
 Source: researchgate.net
Source: researchgate.net
3732 K Boiling point of ethanol. Argon isotope of mass 40. Dichlorodifluoromethane Properties - Properties of saturated liquid Dichlorodifluoromethane R-12 - CCl 2 F 2 - density specific heat capacity kinematic viscosity thermal conductivity and Prandtl number. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. The boiling point temperature will be lower if the atmospheric pressure is decreased.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Argon isotope of mass 40. Examples of uses of distillation include purification of alcohol desalination crude oil refining and making liquefied gases from air. 246046C 410883F 27104 K Block. 56 C 1328 F Boiling point of alcohol. Distillation is the process of separating components of a mixture based on different boiling points.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title boiling point f argon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.