Boiling point dichloromethane
Home » datasheet » Boiling point dichloromethaneBoiling point dichloromethane
Boiling Point Dichloromethane. Dichromic acid H 2 Cr 2 O 7 is the fully protonated form of the dichromate ion and also can be seen as the product of adding chromium trioxide to molecular chromic acid. It is a Lewis acid and can react with a Lewis base such as pyridine in a non-aqueous medium such as dichloromethane Collins reagent. Boiling point of DCM. DCM is used as a solvent in the food industry and as a paint remover.
 Dichloromethane Wikipedia From en.wikipedia.org
Dichloromethane Wikipedia From en.wikipedia.org
The temperature of the column does not have to be above the boiling. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 23 vol Lower 13 vol Vapor Pressure 350 mbar. Product Name Dichloromethane anhydrous Cat No. Ethyl acetate CH 3 COOC 2 H 5 is an excellent solvent with boiling point 78 C 172 F. Boiling point of DCM.
Melting point of DCM-976 0 C.
Hazards of using Dichloromethane. It is a Lewis acid and can react with a Lewis base such as pyridine in a non-aqueous medium such as dichloromethane Collins reagent. Product Name Dichloromethane anhydrous Cat No. Looking for www gmail com different account login. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 23 vol Lower 13 vol Vapor Pressure 350 mbar. DCM is used as a solvent in the food industry and as a paint remover.

The lower the boiling point is the higher the vapor pressure of the compound and the shorter retention time usually is because the compound will spent more time in the gas phase. The compound is also used in the production of aerosol formulations. Melting point of DCM-976 0 C. Ethyl acetate CH 3 COOC 2 H 5 is an excellent solvent with boiling point 78 C 172 F. It is also used as a degreasing agent.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Boiling point of DCM. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample. For instance both DMF and DMSO will boil below 50 C if the vacuum is reduced from 760 torr. Dichromic acid H 2 Cr 2 O 7 is the fully protonated form of the dichromate ion and also can be seen as the product of adding chromium trioxide to molecular chromic acid. Dichromic acid will.
 Source: acs.org
Source: acs.org
The lower the boiling point is the higher the vapor pressure of the compound and the shorter retention time usually is because the compound will spent more time in the gas phase. Looking for www gmail com different account login. As the compound is highly volatile in nature it can cause acute inhalation hazards. The compound is also used in the production of aerosol formulations. For instance both DMF and DMSO will boil below 50 C if the vacuum is reduced from 760 torr.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is a Lewis acid and can react with a Lewis base such as pyridine in a non-aqueous medium such as dichloromethane Collins reagent. As the compound is highly volatile in nature it can cause acute inhalation hazards. It can however be cooled down to 78 C 108 F using a dry. Dichloromethane methylene chloride CH 2 Cl 2 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is too low to make it a good crystallization solvent. The lower the boiling point is the higher the vapor pressure of the compound and the shorter retention time usually is because the compound will spent more time in the gas phase.

Looking for www gmail com different account login. Boiling point of DCM. It is a Lewis acid and can react with a Lewis base such as pyridine in a non-aqueous medium such as dichloromethane Collins reagent. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 23 vol Lower 13 vol Vapor Pressure 350 mbar. Looking for www gmail com different account login.
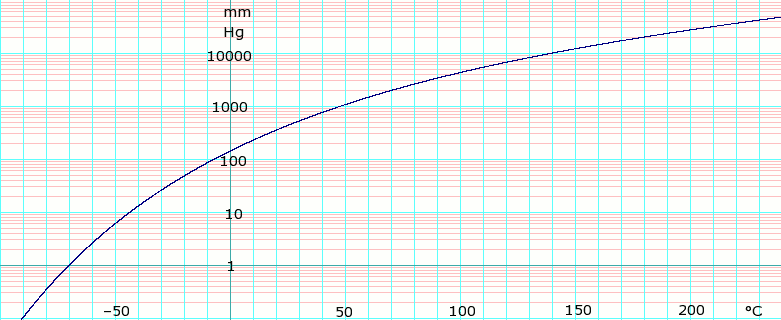 Source: en.wikipedia.org
Source: en.wikipedia.org
The temperature of the column does not have to be above the boiling. Dichloromethane methylene chloride CH 2 Cl 2 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is too low to make it a good crystallization solvent. Dichromic acid will. Melting point of DCM-976 0 C. Product Name Dichloromethane anhydrous Cat No.
 Source: future4200.com
Source: future4200.com
The lower the boiling point is the higher the vapor pressure of the compound and the shorter retention time usually is because the compound will spent more time in the gas phase. Product Name Dichloromethane anhydrous Cat No. Dichromic acid H 2 Cr 2 O 7 is the fully protonated form of the dichromate ion and also can be seen as the product of adding chromium trioxide to molecular chromic acid. Ethyl acetate CH 3 COOC 2 H 5 is an excellent solvent with boiling point 78 C 172 F. The compound is also used in the production of aerosol formulations.
The temperature of the column does not have to be above the boiling. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 23 vol Lower 13 vol Vapor Pressure 350 mbar. Dichromic acid H 2 Cr 2 O 7 is the fully protonated form of the dichromate ion and also can be seen as the product of adding chromium trioxide to molecular chromic acid. DCM is used as a solvent in the food industry and as a paint remover. Dichromic acid will.

The lower the boiling point is the higher the vapor pressure of the compound and the shorter retention time usually is because the compound will spent more time in the gas phase. Melting PointRange-97 C -1426 F Boiling PointRange 39 C 1022 F Flash Point No information available Evaporation Rate No information available Flammability solidgas Not applicable Flammability or explosive limits Upper 23 vol Lower 13 vol Vapor Pressure 350 mbar. It is a Lewis acid and can react with a Lewis base such as pyridine in a non-aqueous medium such as dichloromethane Collins reagent. Dichromic acid will. It can however be cooled down to 78 C 108 F using a dry.
 Source: chegg.com
Source: chegg.com
Melting point of DCM-976 0 C. That is one of the main reasons why low boiling solvents ie diethyl ether dichloromethane are used as solvents to dissolve the sample. Dichloromethane methylene chloride CH 2 Cl 2 is useful as a solvent pair with ligroin but its boiling point 35 C 95 F is too low to make it a good crystallization solvent. The compound is also used in the production of aerosol formulations. The temperature of the column does not have to be above the boiling.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point dichloromethane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.