Boiling point cyclohexane
Home » datasheet » Boiling point cyclohexaneBoiling point cyclohexane
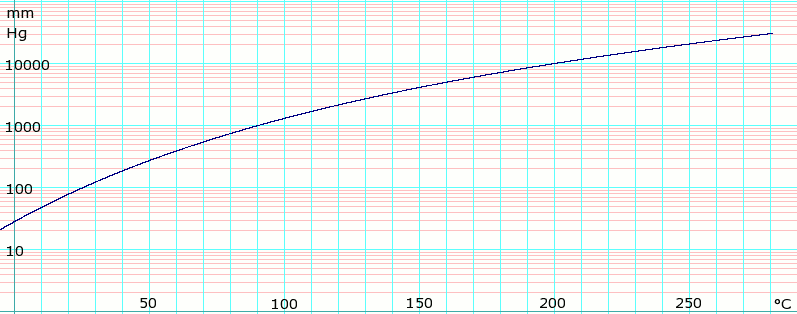
Boiling Point Cyclohexane. The boiling point is specific for the given substanceFor example the boiling point of. For example if we synthesized a known liquid that boiled at 120-122 C this value could be used to confirm that we prepared what we were interested in and that our substance was reasonably pure. Page 5 of 7 MSDS Cyclohexane Vapor Pressure mm Hg. Bromine chloroform cyclohexane ethyl acetate triethylamine.
 Cyclohexane Data Page Wikipedia From en.wikipedia.org
Cyclohexane Data Page Wikipedia From en.wikipedia.org
When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. 26 Ether 1 10. Of course additional criteria must also be satisfied before the. 81C 178F Melting Point. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C.
Page 5 of 7 MSDS Cyclohexane Vapor Pressure mm Hg.
Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. Water boils at 373 K and ethanol boils at. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. 95 20C 68F Evaporation Rate BuAc1. Trans-cyclohexane-12-diol is a cyclohexane-12-diol with trans-configuration.

Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. It has a role as a human xenobiotic metabolite. Each point on this line therefore describes the vapor. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. Page 5 of 7 MSDS Cyclohexane Vapor Pressure mm Hg.

Similarly an azeotropic mixture that has a boiling point lesser than its constituents is known as minimum boiling azeotropes. Acetic acid anhydride CH 3 COO 2 O. The boiling point and boiling point range have been used as criteria in confirming both the identity and purity of a substance. Δt i K b m. Carbon dioxide and carbon monoxide may form when heated to.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Stability and Reactivity Stability. 2 We utilize this formula. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C. 3 Chemical and Physical Properties Expand this section.

Average boiling point from gravity and molecular weight - Formulas and examples of calculation of boiling point of hydrocarbon mixtures from gravity and molecular weight Benzene - Density and Specific Weight - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher. Its not a straightforward topic. Boiling point ºC Dielectric Constant Molecular Weight Acetic Acid-d 4 1165 1 204 5 22 17899 1 200 7 20 115 112 167 118 61 6408 Acetone-d 6 205 5 22 20668 1 2992 7 09 194 28 087 -94 565 207 6412 Acetonitrile-d 3 194 5 25 11869 1 139 7 21 21 084 -45 816 375 4407 Benzene-d 6 716 1 12839 3 243 04 095 55 801 23 8415 Chloroform-d. Boiling Point Elevation and Freezing Point Depression. Search azeotropic data of organic mixtures On this page you can check that a mixture of selected organic substances is zeotropic or azeotropicThe azeotropic information boiling pointtemperature composition is predicted using the UNIFAC modified Dortmund version model.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
DMSO nitrobenzene octanoles sulfuric acid. The definition of the boiling point of a liquid in an open container then is the temperature at which its vapor pressure equals atmospheric pressure. High molecular or high boiling compounds. The boiling point and boiling point range have been used as criteria in confirming both the identity and purity of a substance. 147 g 16214 gmol 0647 kg 140127 m.
 Source: theory.labster.com
Source: theory.labster.com
Maximum Boiling Azeotropes or Positive Azeotrope. Carbon dioxide and carbon monoxide may form when heated to. Note that under vacuum the BP of a liquid will be lower than the BP at atmospheric pressure. DMSO nitrobenzene octanoles sulfuric acid. The boiling point and boiling point range have been used as criteria in confirming both the identity and purity of a substance.
 Source: chegg.com
Source: chegg.com
Boiling Point Elevation and Freezing Point Depression. Highly branched vs. The mole fraction of component 1 in the mixture can be represented by the symbol x 1. Consider for example ethanol consisting of a weight concentration of approximately ninety-five per cent and four per cent of water. According to this CI scale all n-paraffins have a CI value of 0 while cyclohexane the simplest naphthene has a CI value of 50 and benzene has a CI value of 100.

When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. The mole fraction of component 1 in the mixture can be represented by the symbol x 1. Stable at room temperature in sealed containers. Search azeotropic data of organic mixtures On this page you can check that a mixture of selected organic substances is zeotropic or azeotropicThe azeotropic information boiling pointtemperature composition is predicted using the UNIFAC modified Dortmund version model. Some commonly used solvent pairs are water-ethanol acetic.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
147 g 16214 gmol 0647 kg 140127 m. Alcohol - ethyl grain ethanol C. 1 Structures Expand this section. 26 Ether 1 10. Dioxane methanol ethanol nitric acid nitromethane pyridine phosphorous oxychloride.

Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. C I 87 552 T b 4737 S G 4568. 147 g 16214 gmol 0647 kg 140127 m. Water boils at 373 K and ethanol boils at. The definition of the boiling point of a liquid in an open container then is the temperature at which its vapor pressure equals atmospheric pressure.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point cyclohexane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.