Boiling point chloroform
Home » datasheet » Boiling point chloroformBoiling point chloroform
Boiling Point Chloroform. Reactive or incompatible with the following. CAMEO Chemicals-2587 F at 760 mm Hg NTP 1992 National Toxicology Program Institute of Environmental Health Sciences. There can be VLE data for mixtures of four or more components but such a boiling-point diagram is hard to show in either tabular or graphical form. C 2 H 4 O 2.
 Boiling Points Of Mixtures Of Acetic Acid Chloroform And Water P Download Table From researchgate.net
Boiling Points Of Mixtures Of Acetic Acid Chloroform And Water P Download Table From researchgate.net
1 Physical and chemical properties Physical state Molecular weight pH Critical temperature Relative density Vapor pressure Vapor density Volatility Odor threshold Evaporation rate Solubility Odor Color Molecular formula CHCl3 VOC 100. 1084 C 1983 F Melting point of iron. 3275 C 621 F Melting point of silver. Not determined MeltingFreezing point. 1064 C 19475 F Melting point of copper. Alcohol - ethyl grain ethanol C.
The tendency of a given chemical species to partition itself preferentially between liquid and vapor phases is the Henrys law constant.
The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Where K b is the molal boiling point constant and m is the concentration of the solute expressed as molality. The change in the boiling point is calculated from. Solvents like dichloromethane methylene chloride in older literature chloroform diethyl ether or ethyl ester will form two. Not determined Vapor density. In a binary boiling-point diagram.
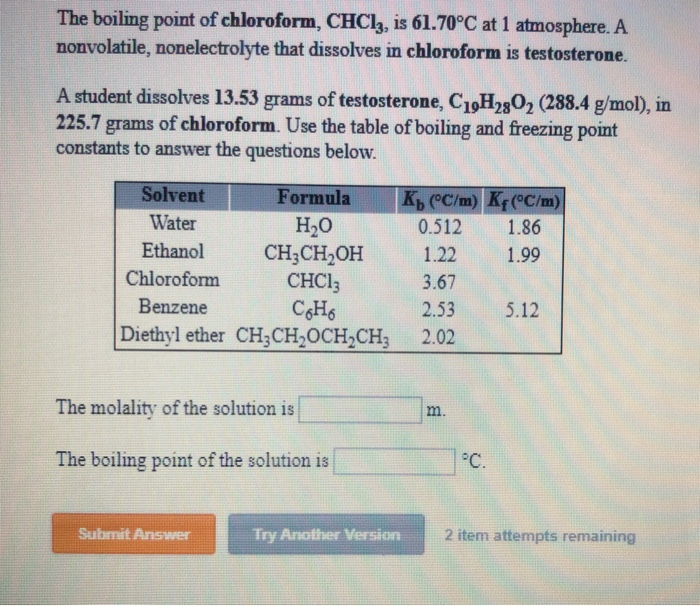 Source: chegg.com
Source: chegg.com
1084 C 1983 F Melting point of iron. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. For such multi-component mixtures as. Δ T b K b m. Aromatic Chloroform Odor Vapor pressure.
 Source: thermopedia.com
Source: thermopedia.com
The tendency of a given chemical species to partition itself preferentially between liquid and vapor phases is the Henrys law constant. Not determined MeltingFreezing point. Alcohol - ethyl grain ethanol C. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. The IUPAC name is trichloroetheneIndustrial abbreviations include TCE trichlor Trike Tricky and tri.
 Source: toppr.com
Source: toppr.com
1538 C 2800 F Melting point of lead. Information on the properties of common solvents used in organic chemistry including boiling points solubility density dielectric constants and flash points. Formerly used as an inhaled anesthetic during surgery the primary use of chloroform today is in industry where it is used as a solvent and in the production of the refrigerant freon. The chemical compound trichloroethylene is a halocarbon commonly used as an industrial solventIt is a clear colourless non-flammable liquid with a chloroform-like sweet smell. Slightly soluble Boiling pointBoiling range.
 Source: doubtnut.com
Source: doubtnut.com
Chemical Hazard Response Information System CHRIS - Hazardous Chemical Data. MW 92 bp 111 C 2318 F. Not determined Vapor density. Common Solvents Used in Organic Chemistry. The melting point is also referred to as liquefaction point solidus or liquidus.
 Source: researchgate.net
Source: researchgate.net
Chemical Hazard Response Information System CHRIS - Hazardous Chemical Data. For such multi-component mixtures as. For example phenol molecular weight MW 94 boiling point bp 182 C 3596 F has a boiling point more than 70 degrees higher than that of toluene C 6 H 5 CH 3. We would like to show you a description here but the site wont allow us. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles.
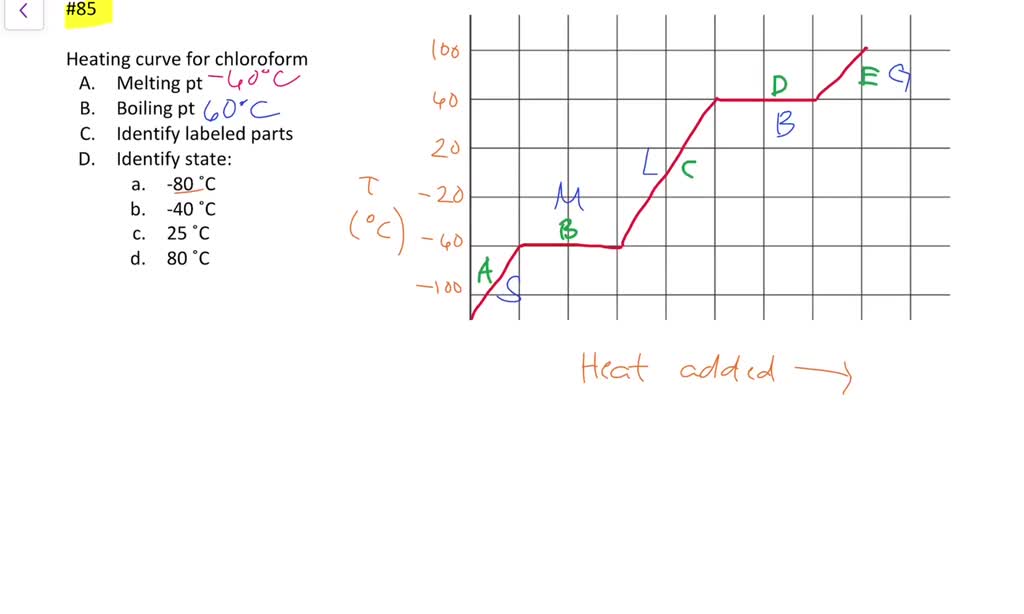 Source: numerade.com
Source: numerade.com
The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Slightly soluble Boiling pointBoiling range. Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles. Where K b is the molal boiling point constant and m is the concentration of the solute expressed as molality.
 Source: ddbst.com
Source: ddbst.com
Aromatic Chloroform Odor Vapor pressure. Δ T b K b m. Chemical Hazard Response Information System CHRIS - Hazardous Chemical Data. 1538 C 2800 F Melting point of lead. A solution boils at a slightly higher temperature than the pure solvent.
 Source: chemicals.ie
Source: chemicals.ie
Acetic acid anhydride CH 3 COO 2 O. 605 - 615C 1409 - 1427F Partition coefficient n-. Not determined MeltingFreezing point. 1 Physical and chemical properties Physical state Molecular weight pH Critical temperature Relative density Vapor pressure Vapor density Volatility Odor threshold Evaporation rate Solubility Odor Color Molecular formula CHCl3 VOC 100. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the.
 Source: researchgate.net
Source: researchgate.net
Reactive or incompatible with the following. Since most of the extractions are performed using aqueous solutions ie 5 NaOH 5 HCl the miscibility of the solvent with water is a crucial point as well as the compatibility of the reagent with the compounds and the solvent of the solution to be extracted. The boiling point data for some solvents are provided in Table 1. Chemical Hazard Response Information System CHRIS - Hazardous Chemical Data. Melting points of common materials Melting point of steel.
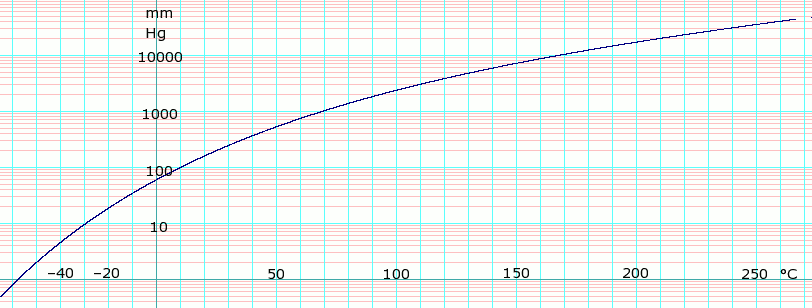 Source: en.wikipedia.org
Source: en.wikipedia.org
Not determined MeltingFreezing point. A similar property of solutions is boiling point elevation. 1538 C 2800 F Melting point of lead. The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Δ T b K b m.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point chloroform by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.