Boiling point ch4
Home » datasheet » Boiling point ch4Boiling point ch4
Boiling Point Ch4. A phaseout and transition to HFO-1234yf and other refrigerants with GWPs similar to CO 2 began in 2012 within the automotive market. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Since the density of water is 1g mL the mass of 120mL is 120g. Only when hydrogen is loaded on a launch vehicle where no refrigeration exists it vents to the atmosphere.
 Boiling Point Trend For Alkanes Youtube From youtube.com
Boiling Point Trend For Alkanes Youtube From youtube.com
CBr4 has 146 compared with 42 in CF4 and 74 in CCl4. Which of the following compounds will be most soluble in pentane C5H12. Of diamond 351 at 20C. What unit of. NH3 a I II and III b II IV and V c I III and IV d I IV and V e II and V 5. At room temperature Ionic Bonds have Solid-state.
Natural Gas - Pipe Sizing - Sizing natural gas pipes - pressures above 5 psi 35 kPa.
The stronger the dispersion forces the higher the boling point will be. Liquid hydrogen is stored and transported without boil-off because helium which has a lower boiling point than hydrogen acts as cooling refrigerant. 0 kJmol Flash Point. A CH3CH2CH2CH2OH B CH2CH2CH2OH C CH3CH2CH2CH3 D CH3CH2CH3 E CH3CH2CH2CH2CH2CH2CH2CH3. B lower because the atmospheric pressure is lower. Temperature given as C F K and R.
 Source: quizlet.com
Source: quizlet.com
15 min for CH4 113 min for octane 147 min for the unknown and 190 min for nonane. The webz says that the boiling point of methane is 16150 C. The main point of using the modnumpy functions is that they work element-wise on elements of an array. The boiling point of water at sea level is 100 C. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
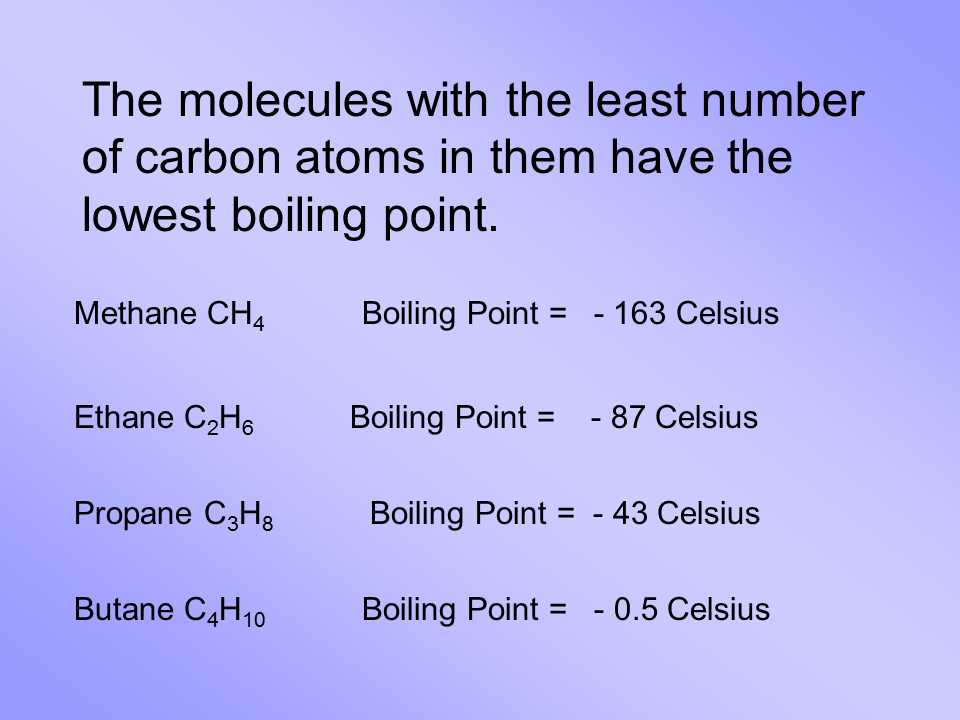
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. The presence of a bond. Covalent Bonds are in Liquid or gaseous State at room temperature. Drawing the Lewis structure for CH 4 named methane requires only single bondsIts one of the easier Lewis structures to draw. Low Polarity and more Flammable.

2 C Index of Refraction. A CH4 b KI c CS2 d HF e I2 6. Distillation is a way to separate mixtures based upon differences in boiling point. Answer 1 of 3. Much more dense than water and insoluble in water.
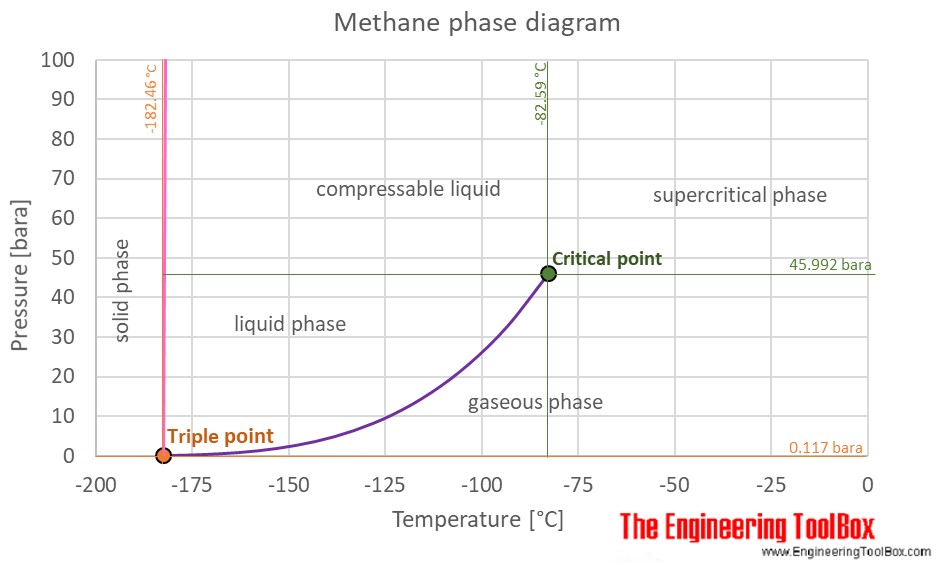 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
It has the formula CF 3 CH 2 F and a boiling point of 263 C 1534 F at atmospheric pressure. Which compound has the lowest boiling point. High Melting Point and Boiling Point. That of carbon tetrachloride is 7672 C. Well BOTH molecules are highly symmetrical and thus have no resultant DIPOL.
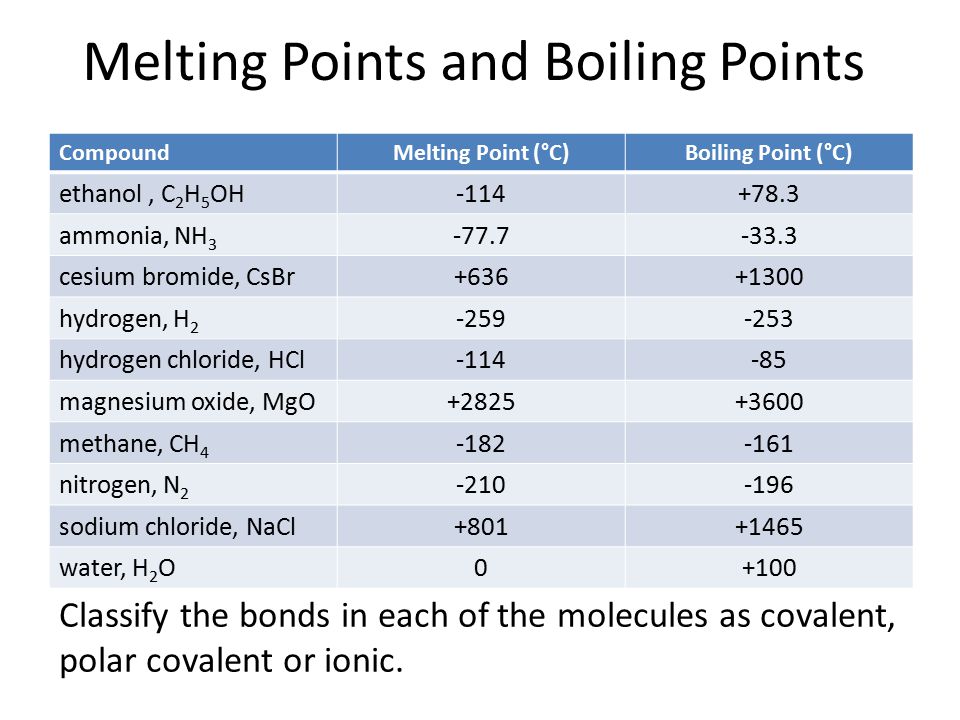 Source: slideplayer.com
Source: slideplayer.com
Natural gas is an odorless gaseous mixture of hydrocarbonspredominantly made up of methane CH4. The webz says that the boiling point of methane is 16150 C. Water Boiling Points at Higher Pressure - Online calculator figures and tables showing boiling points of water at pressures ranging from 147 to 3200 psia 1 to 220 bara. Drawing the Lewis Structure for CH 4. Feb 16 2020 Likewise which has a higher boiling point CCl4 or CBr4.
 Source: youtube.com
Source: youtube.com
The main point of using the modnumpy functions is that they work element-wise on elements of an array. The remaining natural gas is primarily methane with small amounts of other hydrocarbons. CBr4 has 146 compared with 42 in CF4 and 74 in CCl4. CBr4 is the highest boiling point. 2 C Index of Refraction.

You are given a. A phaseout and transition to HFO-1234yf and other refrigerants with GWPs similar to CO 2 began in 2012 within the automotive market. Der Energieinhalt von 1 Nm Wasserstoff entspricht 034 l Benzin 1 l flüssiger Wasserstoff entspricht 027 l Benzin1 kg Wasserstoff entspricht 275 kg Benzin. Methane in general is very stable but mixtures of methane and air with the methane content between 5 and 14 percent by volume are explosive. Because of LNGs relatively high production cost.
 Source: reddit.com
Source: reddit.com
Also Check Difference Between Ionic Covalent and Metallic bonds. High Polarity and less Flammable. Arrange KCl NH3 and CH4 in order of increasing boiling point. Feb 16 2020 Likewise which has a higher boiling point CCl4 or CBr4. The boiling point of water at sea level is 100 C.
 Source: thermopedia.com
Source: thermopedia.com
Only when hydrogen is loaded on a launch vehicle where no refrigeration exists it vents to the atmosphere. Of graphite 226 at 20C. At room temperature Ionic Bonds have Solid-state. Der Energieinhalt von 1 Nm Wasserstoff entspricht 034 l Benzin 1 l flüssiger Wasserstoff entspricht 027 l Benzin1 kg Wasserstoff entspricht 275 kg Benzin. Which of the following compounds will be most soluble in pentane C5H12.
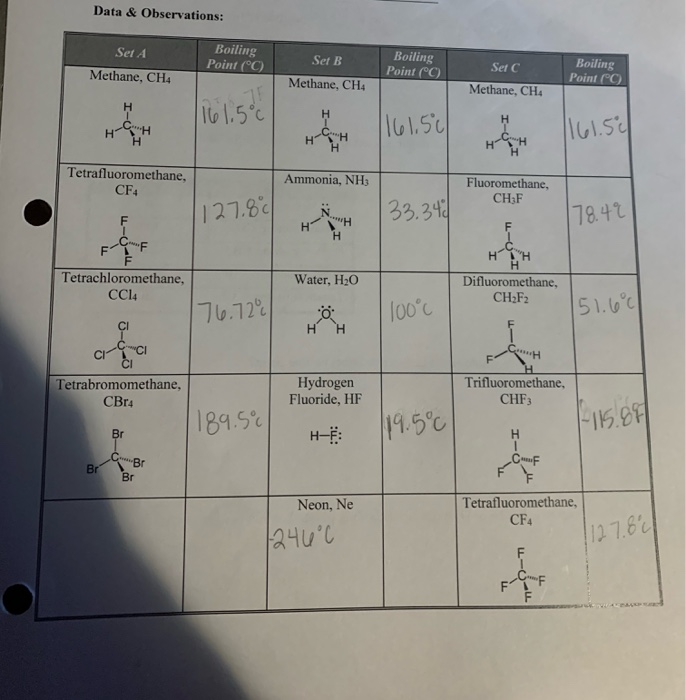 Source: chegg.com
Source: chegg.com
Much more dense than water and insoluble in water. CBr4 has 146 compared with 42 in CF4 and 74 in CCl4. The remaining natural gas is primarily methane with small amounts of other hydrocarbons. R-134a cylinders are colored light blue. Calculate the energy required to heat 120mL of water for a cup of coffee to boiling point if the initial water temperatuer is 200C.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point ch4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.