Boiling point benzene
Home » datasheet » Boiling point benzeneBoiling point benzene
Boiling Point Benzene. Think how confused poor JH. -269 C -452 F. When the temperature of a liquid is below its boiling point we can assume that the only molecules that can escape from the liquid to form a gas are those that lie near the surface of the liquid. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon.
 3 3 Melting Points And Boiling Points Introductory Organic Chemistry From openoregon.pressbooks.pub
3 3 Melting Points And Boiling Points Introductory Organic Chemistry From openoregon.pressbooks.pub
In order for a liquid to boil its vapor pressure needs to exceed ambient pressure which is harder to achieve once you add a nonvolatile component. When a solute is added to the solvent some of the solute molecules occupy the space near the surface of the liquid as shown in the figure below. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon. For hydrocarbons with the same carbon number the boiling point increases in the following order. Transfer a few mL of benzene in the fusion tube. How Boiling Point Elevation Works.
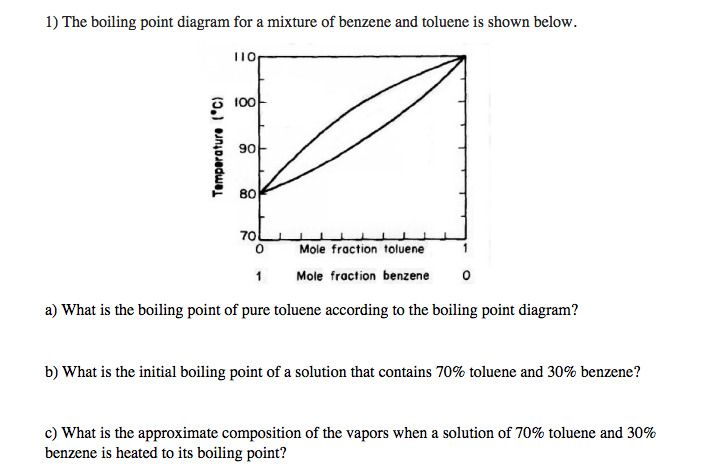
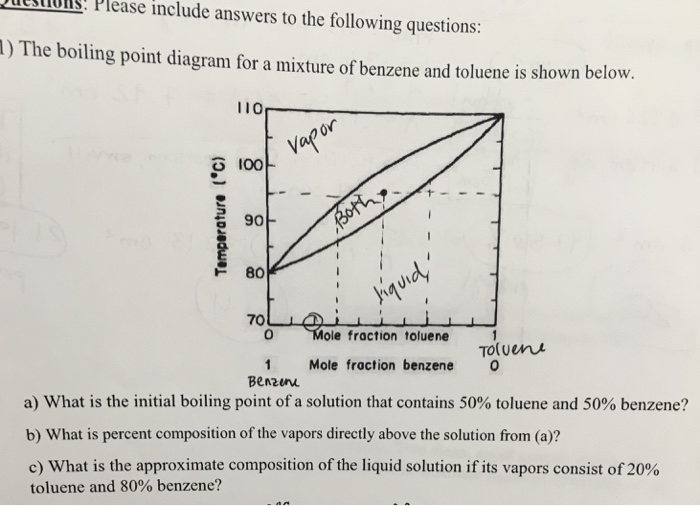
Binary mixture VLE data at a certain overall pressure such as 1 atm showing mole fraction vapor and liquid concentrations when boiling at various temperatures can be shown as a two-dimensional graph called a boiling-point diagram.
100 C 212 F Boiling point of water in Kelvin. Transfer a few mL of benzene in the fusion tube. However if heated it becomes a gas and when cooled it becomes a solid. For hydrocarbons with the same carbon number the boiling point increases in the following order. Benzene is an organic chemical compound with the molecular formula C 6 H 6. 812 o C 801 o C 1253 o Ckgmol-1x02 kg x 11 o C02kg253 o Ckgmol-1 x 00869 moles.
 Source: study.com
Source: study.com
When a solute is dissolved in a solvent the number. -1958 C -3204 F Boiling point of liquid helium. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. The boiling point of pure benzene is 801 o C and its ebullioscopic constant is 253 o Cmolal. This makes sense when you consider that melting involves unpacking the molecules from their ordered array.

Binary mixture VLE data at a certain overall pressure such as 1 atm showing mole fraction vapor and liquid concentrations when boiling at various temperatures can be shown as a two-dimensional graph called a boiling-point diagram. 7837 C 1731 F Boiling point of nitrogen. And then when he got to the right answer I think the joy of figuring it out must have been mixed with some relief. 56 C 1328 F Boiling point of alcohol. When a solute is added to the solvent some of the solute molecules occupy the space near the surface of the liquid as shown in the figure below.
 Source: chegg.com
Source: chegg.com
And then when he got to the right answer I think the joy of figuring it out must have been mixed with some relief. Calculate the boiling point of a 481 by mass. Multisubstituted alkane singelsubstituted alkane singelsubstituted alkene normal alkene normal alkane alkyl cyclohexane alkylbenzene cycloalkene cycloalkane 2- 4- and 3-alkanol 1-alkylnaphthalene 1-alkanol. The acetic acid dimerized in the benzene and formed CH 3 COOH 2. This makes sense when you consider that melting involves unpacking the molecules from their ordered array.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
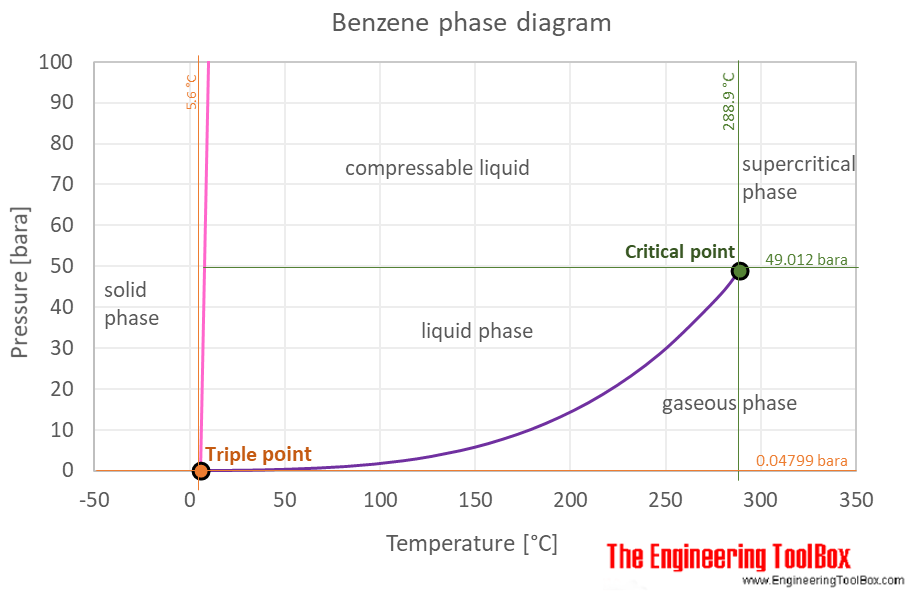
Benzene is a liquid at standard conditions. -1958 C -3204 F Boiling point of liquid helium. In order for a liquid to boil its vapor pressure needs to exceed ambient pressure which is harder to achieve once you add a nonvolatile component. Multisubstituted alkane singelsubstituted alkane singelsubstituted alkene normal alkene normal alkane alkyl cyclohexane alkylbenzene cycloalkene cycloalkane 2- 4- and 3-alkanol 1-alkylnaphthalene 1-alkanol. The acetic acid dimerized in the benzene and formed CH 3 COOH 2.
Benzene is an organic chemical compound with the molecular formula C 6 H 6. The acetic acid dimerized in the benzene and formed CH 3 COOH 2. Rearranging the boiling point equation to yield molality and substituting the molal boiling point constant from Table 1 you can derive the molality of the solution. 7837 C 1731 F Boiling point of nitrogen. Boiling point of water.
 Source: ddbst.com
Source: ddbst.com
And then when he got to the right answer I think the joy of figuring it out must have been mixed with some relief. Binary mixture VLE data at a certain overall pressure such as 1 atm showing mole fraction vapor and liquid concentrations when boiling at various temperatures can be shown as a two-dimensional graph called a boiling-point diagram. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon. Vant Hoff must have been. Due to the cyclic.
 Source: researchgate.net
Source: researchgate.net
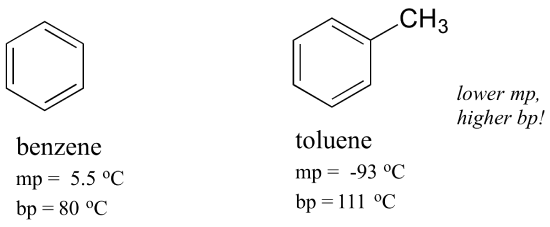
Rearranging the boiling point equation to yield molality and substituting the molal boiling point constant from Table 1 you can derive the molality of the solution. For hydrocarbons with the same carbon number the boiling point increases in the following order. When the temperature of a liquid is below its boiling point we can assume that the only molecules that can escape from the liquid to form a gas are those that lie near the surface of the liquid. And then when he got to the right answer I think the joy of figuring it out must have been mixed with some relief. The key factor for the boiling point trend in this case is size toluene has one more carbon whereas for the melting point trend shape plays a much more important role.
 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
The mole fraction of component 1 in the mixture can be represented by the symbol x 1. And then when he got to the right answer I think the joy of figuring it out must have been mixed with some relief. The phase diagram for benzene shows the phase behavior with changes in temperature and pressure. Benzene is a liquid at standard conditions. When a solute is added to the solvent some of the solute molecules occupy the space near the surface of the liquid as shown in the figure below.

Solvent Boiling Points Chart all boiling points at standard pressure Solvent Boiling Point C Solvent Boiling Point C Acetic Acid 1180 Ethyl Acetate 771 Acetic Acid Anhydride 1390 Ethyl Ether 346 Acetone 563 Ethylene Dichloride 835 Acetonitrile 816 Ethylene Glycol 1975 Benzene 801 Heptane 984 iso-Butanol 1077 n-Hexane 687 n-Butanol 1177 Hydrochloric Acid 848 tert-Butanol. The key factor for the boiling point trend in this case is size toluene has one more carbon whereas for the melting point trend shape plays a much more important role. In order for a liquid to boil its vapor pressure needs to exceed ambient pressure which is harder to achieve once you add a nonvolatile component. When the temperature of a liquid is below its boiling point we can assume that the only molecules that can escape from the liquid to form a gas are those that lie near the surface of the liquid. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each.
 Source: researchgate.net
Source: researchgate.net
Insert the tube in one of the holes of aluminum block and insert the thermometer in the other hole. The curve between the critical point and the triple point shows the benzene boiling point with changes in pressure. From the boiling point elevation formula the following relationship can be obtained. Think how confused poor JH. In a nutshell boiling point increases because most of the solute particles remain in the liquid phase rather than enter the gas phase.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title boiling point benzene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.