Benzene boiling points
Home » datasheet » Benzene boiling pointsBenzene boiling points
Benzene Boiling Points. Phenols are the organic compounds containing benzene ring bonded to a hydroxyl group. All boiling points below are normalatmospheric boiling points. They give the temperature at which the vapor pressure of the liquid is equal to atmospheric pressure at sea. The phase diagram for benzene shows the phase behavior with changes in temperature and pressure.

Individual data points Quantity Value Units Method Reference Comment. Type 15 in the second left field and 18 will appear in the second right field. Lets have a closer look. They are also known as carbolic acids. Methanal is a gas boiling point -21C and ethanal has a boiling point of 21C. Benzene is a liquid at standard conditions.
Graphs show boiling point curves based on temperature and.
Individual data points Quantity Value Units Method Reference Comment. The size of the boiling point is governed by the strengths of the intermolecular. Boiling Point Elevation and Freezing Point Depression. In addition they also lack dipole-dipole interactions since there is no polar covalent bond present. Branched — more sphere-like – better stacking – higher melting point highly branched vs. Benzene is an organic chemical compound with the molecular formula C 6 H 6.
 Source: study.com
Source: study.com
The size of the boiling point is governed by the strengths of the intermolecular. Rearranging the boiling point equation to yield molality and substituting the molal boiling point constant from Table 1 you can derive the molality of the solution. Brake Fluid Dot 4 Dry - Wet boiling points 230 - 155. The other aldehydes and the ketones are liquids with boiling points rising as the molecules get bigger. Branching decreases boiling point.
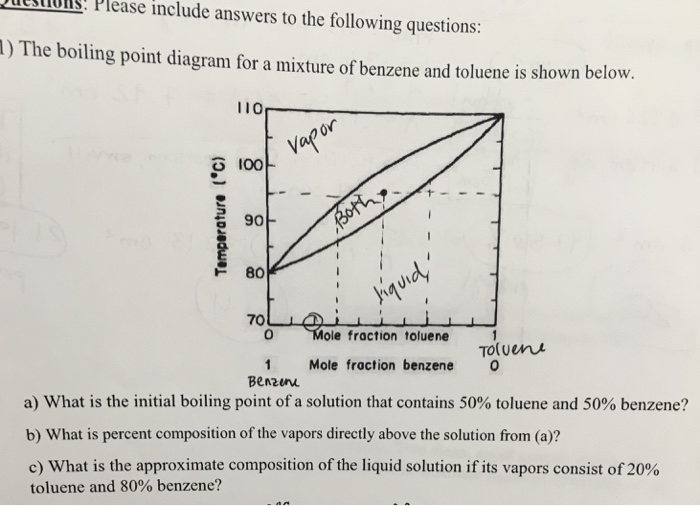
The Relative Strength Of The Four Intermolecular Forces. The phase diagram for benzene shows the phase behavior with changes in temperature and pressure. It also shows. Brake Fluid Dot 3 Dry - Wet boiling points Wet includes hygroscopic moisture 205 - 140. Boiling points on the other hand essentially reflect the kinetic energy needed to release a molecule from the cooperative attractions of the liquid state so that it becomes an unencumbered and relative independent gaseous state species.
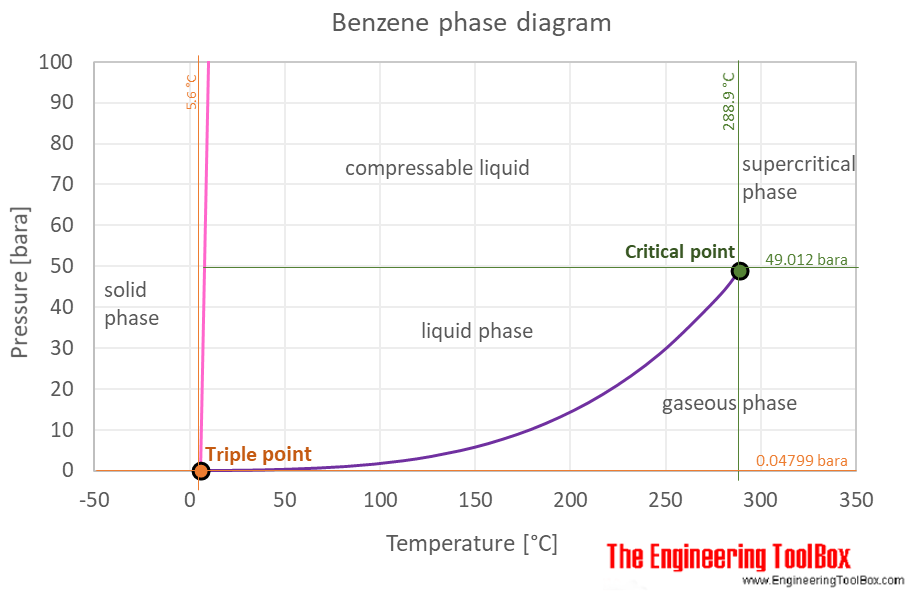 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Individual data points Quantity Value Units Method Reference Comment. They exhibit unique physical and chemical properties in comparison to alcohol. In some cases the table only shows one liquid and the boiling point at various pressures. They are also known as carbolic acids. Branched —more sphere-like - - lower surface area — lower boiling point.
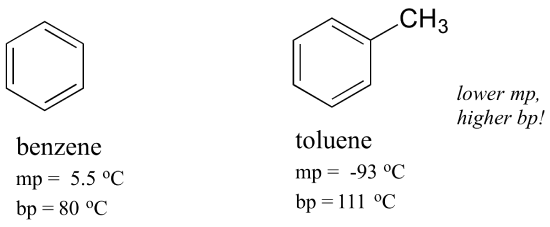 Source: openoregon.pressbooks.pub
Source: openoregon.pressbooks.pub
To use the nomograph given the normal boiling point simply. Benzene -11 1 8 995 2213 80 Butanone -3 18 10 95 79 Carbon disulphide -30 13 50 395 1343 46 Cyclohexane -18 1 9 1027 225 81 Highly Flammable Liquid substances and preparations which have a flash point lower than 0 C and a boiling point or in the case of a boiling range the initial boiling point lower than or equal to 35 C. This will most likely be the case for all problems you encounter related to freezing point depression and boiling point elevation in this course but it is a good idea to keep an eye out for ions. Methanal is a gas boiling point -21C and ethanal has a boiling point of 21C. Brake Fluid Dot 4 Dry - Wet boiling points 230 - 155.
 Source: researchgate.net
Source: researchgate.net
Methanal is a gas boiling point -21C and ethanal has a boiling point of 21C. It has 4 electrons in its outer orbit. Column c is in mm of mercury. 1179 307 166 390 K b K f. A nomograph used to estimate boiling points at reduced pressures.

801 265 55 512 K b K f. The figure below shows the consequences of the fact that solutes lower the vapor pressure of a solvent. Branched —more sphere-like - - lower surface area — lower boiling point. Binary Azeotropic Mixtures - Minimum Boiling Point Substances in mixture. Network solids are extremely hard compounds with very high melting and boiling points due to their endless 3-dimensional network of covalent bonds.
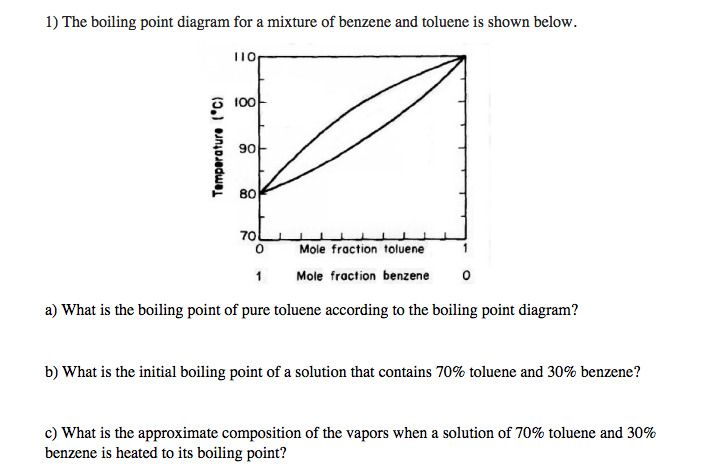 Source: chegg.com
Source: chegg.com
However if heated it becomes a gas and when cooled it becomes a solid. This will most likely be the case for all problems you encounter related to freezing point depression and boiling point elevation in this course but it is a good idea to keep an eye out for ions. Due to the cyclic. Column c is in mm of mercury. They are also known as carbolic acids.
 Source: researchgate.net
Source: researchgate.net
In other cases several liquids at different pressures may be shown. You can now roughly evaluate its boiling point. Boiling points on the other hand essentially reflect the kinetic energy needed to release a molecule from the cooperative attractions of the liquid state so that it becomes an unencumbered and relative independent gaseous state species. To use place a straight edge on two of the three known properties and read out the third. Column c is in mm of mercury.
 Source: ddbst.com
Source: ddbst.com
This makes sense when you consider that melting involves unpacking the molecules from their ordered array. Methanal is a gas boiling point -21C and ethanal has a boiling point of 21C. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon. 1179 307 166 390 K b K f. It has 4 electrons in its outer orbit.

However if heated it becomes a gas and when cooled it becomes a solid. The Role Of Symmetry or lack thereof On Melting And Boiling. Linear versus branched — higher meltingboiling points due to better stacking and surface area contact. Water boils at 18C under 15 millimeters. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title benzene boiling points by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.