Aspirin melting point
Home » datasheet » Aspirin melting pointAspirin melting point
Aspirin Melting Point. What is the Rf value for acetylsalicylic acid. To do the test get 4 test tubes. Also your melting point range may be. Determine start and end temperature of melting.

Enrique Peacock Created Date. This spectrum showed a broad peak at. If the melting point of the sample is unknown or unavailable a fast run with the. Questions answer in. Physical Properties of Aspirin It Shows Following Physical Properties Its molar mass is 18016 gmol. This agent exhibits analgesic antipyretic and anticoagulant properties.
The results displayed a percent yield of 343 from a theoretical yield of about 197g of.
If phenol is present in a sample the resulting color means the product is impure. An esterification reaction is the general name for a chemical reaction in which two reactants typically an alcohol and an acid form an ester as the. Natural mixed triglycerides have somewhat lower melting points the melting point of lard being near 30 º C whereas olive oil melts near -6 º C. Add an acyl group to an oxygen of salicylic acid. Apply the previously learned technique of melting point determination. To do the test get 4 test tubes.

Add a few crystals of salicylic acid to one test tube. You will purify the unknown by recrystallization and determine which of the. Then determine the melting point of aspirin using necessary apparatus. The melting point of pure aspirin is 135C and the melting point of salicylic acid is 158C. Determine start and end temperature of melting.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
3 GCMS screw a cap on the GCMS vial filled with your AspirinEthanol and close tightly. 134 C TCI A2262. Its acid dissociation constant pK a is 35 at 25 C 77 F. Add an acyl group to an oxygen of salicylic acid. Add a few crystals.
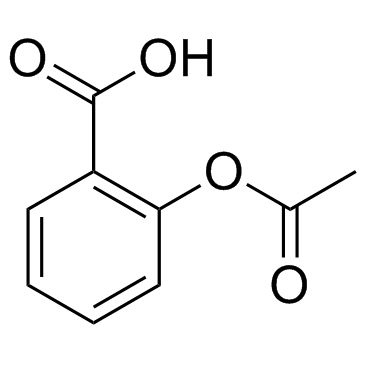 Source: chemsrc.com
Source: chemsrc.com
What is the minimum amount of free salicylic acid allowed in commercial aspirin. Determine start and end temperature of melting. Pure aspirin has a melt point of 135 o C. Uses of AspirinAcetylsalicylic acid-C 9 H 8 O 4 Acetylsalicylic acid acts as an inhibitor of cyclo oxygenase. Aspirin Purity Testing 5.
 Source: studylib.net
Source: studylib.net
Felix Hoffmann a German chemist produced a stable form of acetylsalicylic acid more commonly known as aspirin. Compound MW Amount Needed mmol mp bp Density η D msds. Felix Hoffmann a German chemist produced a stable form of acetylsalicylic acid more commonly known as aspirin. 135 C Jean-Claude Bradley Open Melting Point Dataset 22166 27755 27756 27757. Data and Post-lab Assignment for Lab 2.
 Source: youtube.com
Source: youtube.com
1385 C Jean-Claude Bradley Open Melting Point Dataset 17267. A small melting point range close to the standard of acetylsalicylic acid would suggest a high purity sample meaning the recrystallization process was effective. The synthesis of aspirin is classified as an esterification reaction. The results displayed a percent yield of 343 from a theoretical yield of about 197g of. The purple color indicates the presence of a phenol group.

Enrique Peacock Created Date. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid. Its acid dissociation constant pK a is 35 at 25 C 77 F. Scanning of the temperature until melting occurs. Aspirin Purity Testing 5.
 Source: uchemchemicals.com
Source: uchemchemicals.com
The methods used included recrystallization and scratching to produce a precipitate which was then filtered to remove any excess moisture. Permanent dipole permanent dipole bonds may also be formed from the CO bonds on the aspirin molecule. Impurities have a dramatic influence on the melting points of organic compounds. This is a demonstration of. 139 C Jean-Claude Bradley Open Melting Point Dataset 15219.
Determination of the melting point can also give an indication of the purity of a compound as the presence of impurities lowers the melting point and broadens its melting temperature range. Aspirin is prepared by the O-acylation ie. 3 GCMS screw a cap on the GCMS vial filled with your AspirinEthanol and close tightly. The percent yield was about 767 whereas the temperature range is between 1342 to 1361 ÌŠÌŠC. 139 C Jean-Claude Bradley Open Melting Point Dataset 15219.
 Source: packerintersections.com
Source: packerintersections.com
For example while pure salicylic acid melts sharply at 157 159 o C a sample of wet salicylic acid water is the impurity may melt in the range 145 155 o C. Determination of Purity Phenols form a colored complex with the ferric ion. If phenol is present in a sample the resulting color means the product is impure. For detailed mechanism you may visit Mechanism of Acetylsalicylic acid. If the melting point of the sample is unknown or unavailable a fast run with the.
 Source: vernier.com
Source: vernier.com
Pure aspirin has a melt point of 135 o C. 3g of aspirin can dissolve in 1 liter of water. Add a few crystals. Acetyl salicylic acid aspirin 18016— 135. 134 C TCI A2262.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title aspirin melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.