Argon boiling point
Home » datasheet » Argon boiling pointArgon boiling point
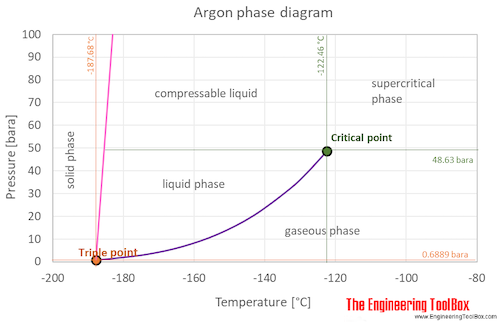
Argon Boiling Point. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. As an example from sodium to argon in third period. 23 K to 353. The argon phase diagram shows the phase behavior with changes in temperature and pressure.
 Argon Thermophysical Properties From engineeringtoolbox.com
Argon Thermophysical Properties From engineeringtoolbox.com
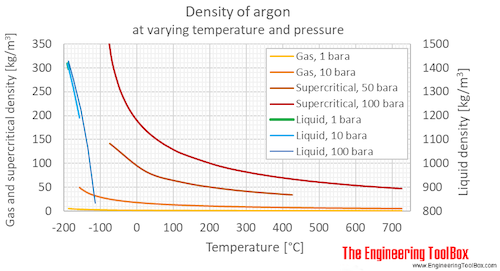
100 C 373 K K C 273 eg. 185848C 302526F 87302 K Block. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. Shield Gas Inerting Fire Extinguishing various others NAME AND ADDRESS. Information about various chemical compounds and elements. Argon molecules are just single argon atoms Ar.
Well BOTH molecules are highly symmetrical and thus have no resultant DIPOL.
Argon isotope of mass 40. The boiling point observed for the water-methanol mixture was much higher than that of pure methanol since water has a much higher boiling point and lower vapor pressure than methanol. Argon is produced industrially by the fractional distillation of liquid air. Argon is a gas at standard conditions. Energy Forms. Click on any elements name for further chemical properties environmental data or health effects.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
There are many reasons to effect for melting and boiling points of elements and compounds. Incompatibility with Other Materials. Now why the disparity. However at low temperature andor high pressures the gas becomes a liquid or a solid. Answer 1 of 3.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
It also shows the saturation pressure with changes in temperature. Chemical name Argon Suppliers details. Finally a point temperature is. That of carbon tetrachloride is 7672 C. Click on any elements name for further chemical properties environmental data or health effects.
 Source: britannica.com
Source: britannica.com
Click on any elements name for further chemical properties environmental data or health effects. The boiling point temperature will be lower if the atmospheric pressure is decreased. Argon gas CHEMICAL FORMULA. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. F Melting point NA Solubility in water vv at 20 C 00337 Specific gravity liquid NA Molecular weight 3995 Section 10.
 Source: researchgate.net
Source: researchgate.net
F Melting point NA Solubility in water vv at 20 C 00337 Specific gravity liquid NA Molecular weight 3995 Section 10. Should you not have quoted the normal boiling points of the 2 solvents. Then it starts to decrease melting and boiling points from VA group to noble gases VIIIA. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. So isobutane is a slightly better choice in cold weather but propane is the best at -42C -44F.
 Source: socratic.org
Source: socratic.org
That of carbon tetrachloride is 7672 C. Stability and Reactivity return to contentsContents Chemical Stability. 23 K to 353. Argon Ar - Argon is a chemical element with the symbol Ar and atomic number 18. 185848C 302526F 87302 K Block.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Then it starts to decrease melting and boiling points from VA group to noble gases VIIIA. State at 20C. However since argon is 38 more dense than air it present an asphyxiation risk because it can displace oxygenated air in enclosed spaces. In the periodic table above black squares indicate elements which are solids at room temperature about 22ºC those in blue squares are liquids at room temperature and those in red squares are gases at room temperature. Relative density gas air1 138.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external atmospheric pressure. Chlorine Cl 2 is a much smaller molecule with comparatively weak van der Waals attractions and so chlorine will have a lower melting and boiling point than sulphur or phosphorus. Know the Uses of Argon Chemical Properties of. Atomic number - Name alphabetically-269. That of carbon tetrachloride is 7672 C.
 Source: en.wikipedia.org
Source: en.wikipedia.org
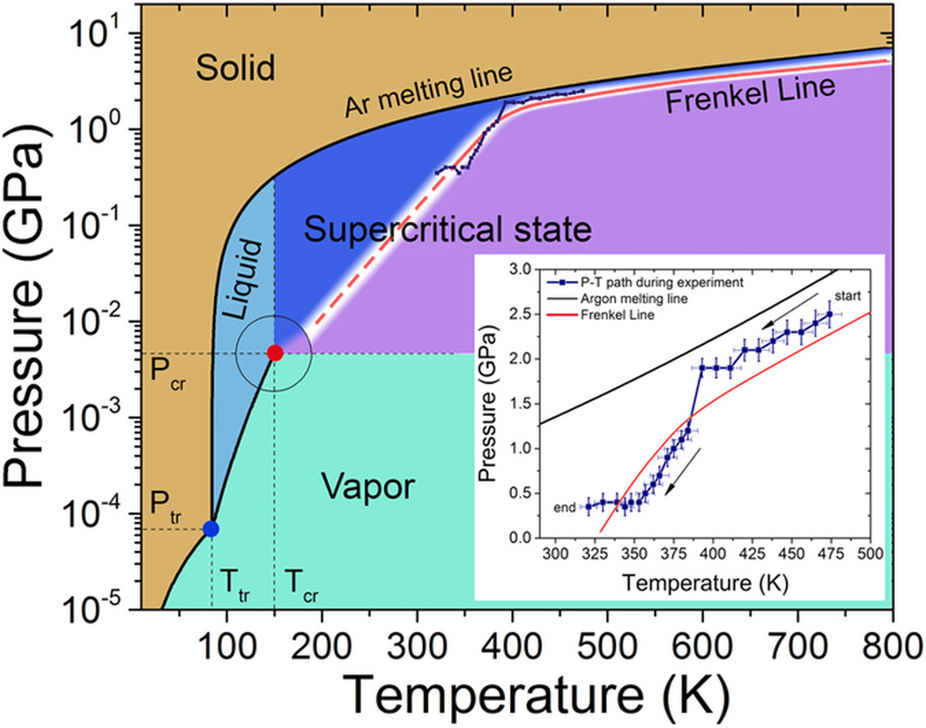
Refrigeration Oxygen Co. Shield Gas Inerting Fire Extinguishing various others NAME AND ADDRESS. PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME. Argon MSDS Material Safety Data Sheet for Argon Ar. The triple point of a substance is the temperature and pressure at which the three phases gas liquid and solid.
 Source: researchgate.net
Source: researchgate.net
Argon molecules are just single argon atoms Ar. At the critical point there is no change of state when pressure is increased or if heat is added. The chemical elements of the periodic chart sorted by. It is associated with liquids and gases. Argon Product use SyntheticAnalytical chemistry.
 Source: rsc.org
Source: rsc.org
Boiling point -1224 deg. Answer 1 of 3. That of carbon tetrachloride is 7672 C. 100 C 373 K K C 273 eg. The graph shows how melting points and boiling points vary across period 3.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title argon boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.