Aniline melting point
Home » datasheet » Aniline melting pointAniline melting point
Aniline Melting Point. Soften melt and flow upon heating eg LDPE HDPE PP PS PVC Nylon PMMA PC ABS PET Characteristics. Distillation is recommended in the case of liquids see Appendix 3. 158F flash point Water-insoluble and somewhat denser than water. 2 Melting point or softening point - i Melting point.
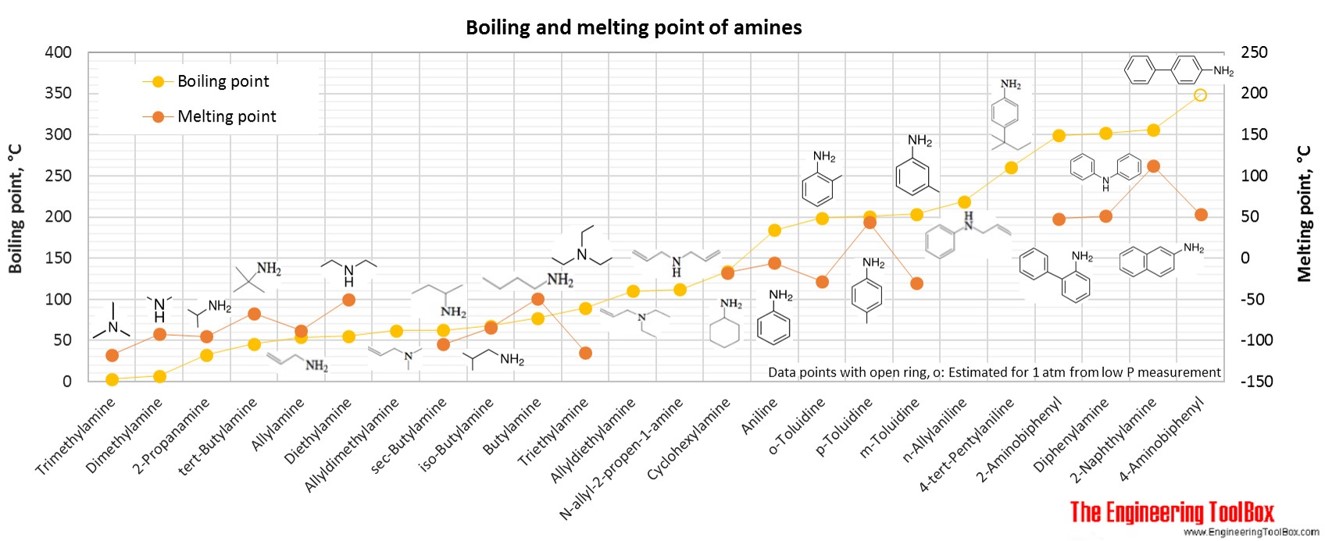 Organic Nitrogen Compounds Physical Data From engineeringtoolbox.com
Organic Nitrogen Compounds Physical Data From engineeringtoolbox.com
Amine any member of a family of nitrogen-containing organic compounds that is derived either in principle or in practice from ammonia NH3. A high aniline point suggests a. Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. -269 C -452 F. 60-09-3 - QPQKUYVSJWQSDY-CCEZHUSRSA-N - Aniline Yellow - Similar structures search synonyms formulas resource links and other chemical information. The reaction of aniline with acetic anhydride is a transformation in which products acetanilide and acetic acid are obtained.
Aniline hydrochloride appears as a white to greenish colored crystalline solid.
Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. Toxic by ingestion and a skin and eye irritant. The reaction of aniline with acetic anhydride is a transformation in which products acetanilide and acetic acid are obtained. 7837 C 1731 F Boiling point of nitrogen. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. 8 THERMOPLASTIC POLYMERS Thermoplastic polymers.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The information in this chart has been supplied to Cole-Parmer by other reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibility. Analysis for elements present. 647 C 1485 F Boiling point of acetone. The distance between molecules in a crystal lattice is small and regular with intermolecular forces serving to constrain the motion of the molecules more severely than in the liquid state. 8 THERMOPLASTIC POLYMERS Thermoplastic polymers.
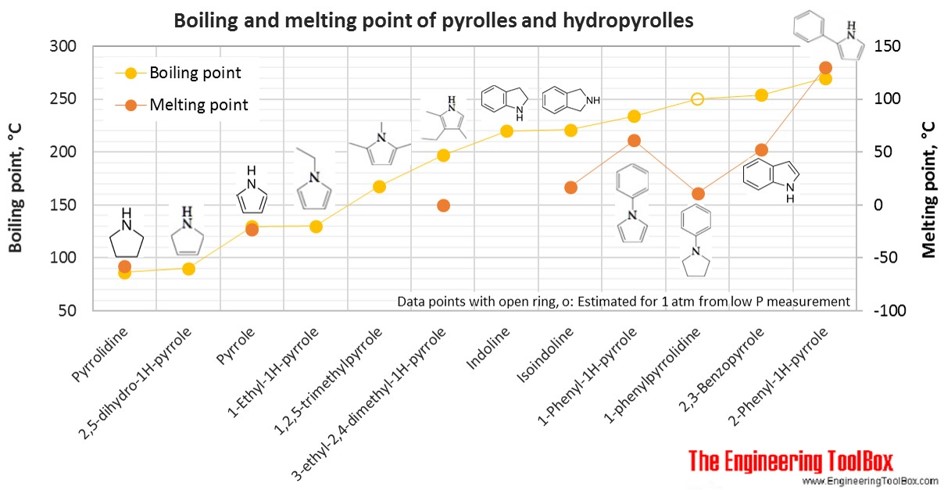 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
-1958 C -3204 F Boiling point of liquid helium. 647 C 1485 F Boiling point of acetone. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. Thermodynamics - Effects of work heat and energy on systems. The melting point is specific for a given substance.

The reaction of aniline with acetic anhydride is a transformation in which products acetanilide and acetic acid are obtained. Aniline is used to make a wide variety of products such as polyurethane foam agricultural chemicals synthetic dyes antioxidants stabilizers for the rubber industry herbicides. Analysis for elements present. A high aniline point suggests a. The melting point depends on the pressure.
647 C 1485 F Boiling point of acetone. Solids prepared in this manner serve a derivative whose. The reaction of aniline with acetic anhydride is a transformation in which products acetanilide and acetic acid are obtained. Molecular size is important but shape is also. The density of aniline is 0989 gml 98 pure and the density of carbon disulphide is1266 gml 99 pure.

Distillation is recommended in the case of liquids see Appendix 3. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. The information in this chart has been supplied to Cole-Parmer by other reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibility. MW 92 bp 111 C 2318 F. Its main use is in the manufacture of precursors to polyurethane dyes and other industrial chemicals.
 Source: chemsynthesis.com
Source: chemsynthesis.com
The melting point shall be determined by ASTM method D2117-82 Standard Test Method for Melting Point of Semicrystalline Polymers by the Hot Stage Microscopy Method which is incorporated by reference. The melting point shall be determined by ASTM method D2117-82 Standard Test Method for Melting Point of Semicrystalline Polymers by the Hot Stage Microscopy Method which is incorporated by reference. Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions. The distance between molecules in a crystal lattice is small and regular with intermolecular forces serving to constrain the motion of the molecules more severely than in the liquid state. Melting Point C 190-220 210-240 Property LDPE HDPE.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The reaction of aniline with acetic anhydride is a transformation in which products acetanilide and acetic acid are obtained. The availability of this incorporation by reference is given in paragraph d1 of. Because aniline is an aromatic molecule that combines readily with other aromatic compounds a low aniline point suggests a low diesel index. It is also a temperature at which a solid crystal turns into a liquid. We say that such a body melts.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The melting point depends on the pressure. It is produced on a large scale from benzene as a precursor to anilineIn the laboratory it is occasionally used as a solvent especially for electrophilic reagents. A solid product is often desirable since it may be recrystallized and a melting point determined. MW 92 bp 111 C 2318 F. 56 C 1328 F Boiling point of alcohol.
 Source: srjng88.medium.com
Source: srjng88.medium.com
Thermodynamics - Effects of work heat and energy on systems. Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Aniline is an organic compound with the formula C 6 H 5 NH 2Consisting of a phenyl group attached to an amino group aniline is the simplest aromatic amineIt is an industrially significant commodity chemical as well as a versatile starting material for fine chemical synthesis. Toxic by ingestion and a skin and eye irritant.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Who are the experts. The catecholamine neurotransmitters ie dopamine epinephrine. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. Which of the two complexes would you expect to have higher melting point and justify your answer. Amine any member of a family of nitrogen-containing organic compounds that is derived either in principle or in practice from ammonia NH3.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title aniline melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.