Aniline boiling point
Home » datasheet » Aniline boiling pointAniline boiling point
Aniline Boiling Point. By weight in mixture. Denser than water 85 lb gal and slightly soluble in water. Toxic by ingestion and a skin and eye irritant. V The solvent should moderately be volatile so crystals dried readily.
 Boiling Points Of Various Fluids At 1 Atm 16 Download Scientific Diagram From researchgate.net
Boiling Points Of Various Fluids At 1 Atm 16 Download Scientific Diagram From researchgate.net
36 g100 mL at 20 C Vapor pressure. The boiling point of organic compounds can give important information about their physical properties and structural characteristics. Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. Aniline is a musty fishy-smelling yellowish to brownish greasy liquid. Produces toxic oxides of nitrogen during combustion.
Aniline dye comes as a powder that you dissolve in water alcohol or petroleum solvents.
Nitrobenzene is an organic compound with the chemical formula C 6 H 5 NO 2It is a water-insoluble pale yellow oil with an almond-like odorIt freezes to give greenish-yellow crystals. V The solvent should moderately be volatile so crystals dried readily. By weight in mixture. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Aniline is an organic compound with the formula C 6 H 5 NH 2. 36 g100 mL at 20 C Vapor pressure.
 Source: researchgate.net
Source: researchgate.net
18413 C 36343 F. 06 mmHg 20 C Acidity pK a. 158F flash point Water-insoluble and somewhat denser than water. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. Of course boiling point relationships may be dominated by even stronger attractive forces such as those involving electrostatic attraction between oppositely charged ionic species and between the partial charge separations of molecular dipoles.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Because aniline is an aromatic molecule that combines readily with other aromatic compounds a low aniline point suggests a low diesel index. A liquid boils when its vapour pressure is equal to the atmospheric pressure. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. V The solvent should moderately be volatile so crystals dried readily. All boiling points below are normalatmospheric boiling points.
 Source: srjng88.medium.com
Source: srjng88.medium.com
Pigmented stains which some people characterize as thinned paints may mask the woods figure and can lend wood a muddy look. Binary Azeotropic Mixtures - Minimum Boiling Point Substances in mixture. Boiling Point and Water Solubility. The boiling point is specific for the given substanceFor example the boiling point of. 36 g100 mL at 20 C Vapor pressure.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is produced on a large scale from benzene as a precursor to anilineIn the laboratory it is occasionally used as a solvent especially for electrophilic reagents. Boiling point helps identify and characterise a compound. Mentor April 13 2020 at 438 pm. Evaporation DMF aniline toluene CALIBRATION. Nitrobenzene is an organic compound with the chemical formula C 6 H 5 NO 2It is a water-insoluble pale yellow oil with an almond-like odorIt freezes to give greenish-yellow crystals.
 Source: researchgate.net
Source: researchgate.net
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Evaporation DMF aniline toluene CALIBRATION. Aniline dye comes as a powder that you dissolve in water alcohol or petroleum solvents. It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils.
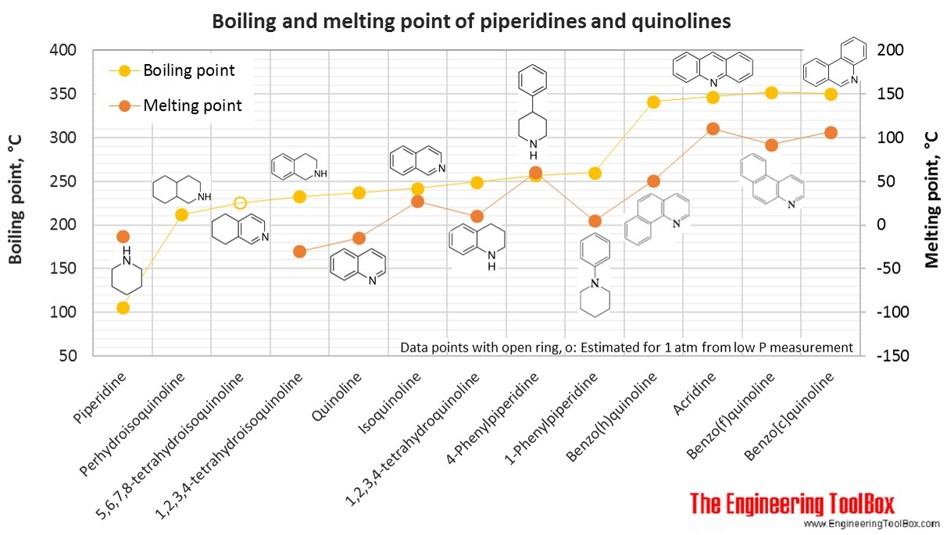 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
The boiling point at atmospheric pressure 147 psia 1 bar absolute for some common fluids and gases can be found from the table below. Aniline hydrochloride appears as a white to greenish colored crystalline solid. Vapour pressure is determined by the kinetic energy of a molecule. 45728 K Solubility in water. Aniline is a musty fishy-smelling yellowish to brownish greasy liquid.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Nitrobenzene is an organic compound with the chemical formula C 6 H 5 NO 2It is a water-insoluble pale yellow oil with an almond-like odorIt freezes to give greenish-yellow crystals. III The crude crystals should not react with the solvent IV The solvent should boil at temperature below the solid melting point. Rahul kumar August 12 2019 at 954 am. Aniline dyes offer an attractive finishing choice today. Binary Azeotropic Mixtures - Minimum Boiling Point Substances in mixture.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Denser than water 85 lb gal and slightly soluble in water. The boiling point is specific for the given substanceFor example the boiling point of. Aniline dyes offer an attractive finishing choice today. A high aniline point suggests a. Physical Property Value Units Temp deg C Source.
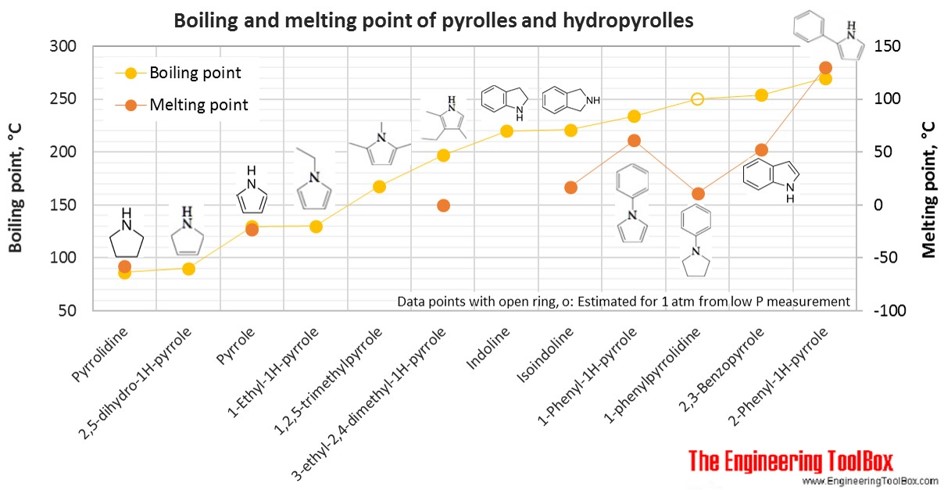 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Acetic acid anhydride CH 3 COO 2 O. Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. Produces toxic oxides of nitrogen during combustion. Stacy November 28 2019 at 1259 am. V The solvent should moderately be volatile so crystals dried readily.
Produces toxic oxides of nitrogen during combustion. It is produced on a large scale from benzene as a precursor to anilineIn the laboratory it is occasionally used as a solvent especially for electrophilic reagents. Molecules having a permanent dipole moment should therefore have higher boiling points than equivalent nonpolar compounds as illustrated by the. Product Boiling Point at Atmospheric Pressure o C Acetaldehyde CH 3 CHO. Produces toxic oxides of nitrogen during combustion.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title aniline boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.