Anhydrous sodium sulfate melting and boiling point
Home » datasheet » Anhydrous sodium sulfate melting and boiling pointAnhydrous sodium sulfate melting and boiling point
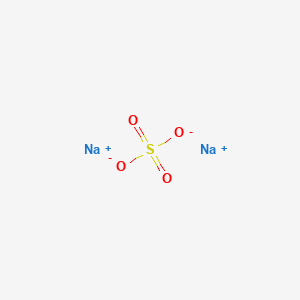
Anhydrous Sodium Sulfate Melting And Boiling Point. Anhydrous insoluble in ethanol. Magnetic susceptibility χ 108010 6 cm 3 mol. In laboratories sodium sulfite is generally prepared from the reaction between gaseous sulfur dioxide SO 2 and sodium hydroxide NaOH. Anhydrous sodium sulfate is a white crystalline solid also known as the mineral thenardite.
 Sodium Sulfate 99 Extra Pure Anhydrous Thermo Scientific Other Inorganic Compounds Chemicals Fisher Scientific From fishersci.co.uk
Sodium Sulfate 99 Extra Pure Anhydrous Thermo Scientific Other Inorganic Compounds Chemicals Fisher Scientific From fishersci.co.uk
Sodium Sulfate Anhydrous Safety Data Sheet according to Federal Register Vol. Draw off the aqueous layer. Another way to dry an ether layer is to wash it with saturated NaCl solution brine before adding drying agent. 6 teaspoons of Sodium sulfate 4. 436 g100 mL 0 C 45 g100 mL 7 C 529 g100 mL 20 C 105 g100 mL 96 C Solubility. Put 15 mL of coffee and tea solution in a separatory funnel 3.
Add 05 g of anhydrous sodium sulfate to the combined dichloromethane extracts in the 25 ml Erlenmeyer flask.
11 04112018 EN English US Page 1 SECTION 1. Sodium Sulfate Na2SO4 -Sodium sulfate is the sodium salt of sulfuric acid. In laboratories sodium sulfite is generally prepared from the reaction between gaseous sulfur dioxide SO 2 and sodium hydroxide NaOH. Draw off the aqueous layer. 15 mL Erlenmeyer flask 5. Large modern plants also produce coarse crystals of Chile saltpeter which do not require further processing by melting.
Swirl the contents of the flask. Anhydrous insoluble in ethanol. Pour the remaining ethyl acetate layer out the neck of the funnel into a 125 mL Erlenmeyer flask add a few grams of anhydrous sodium sulfate swirl occasionally for 5 minutes or so. The brine transfers the water from the ether layer to the aqueous layer. Identification Product form.
 Source: scbt.com
Source: scbt.com
Sodium Sulfate Anhydrous CAS-No. The depletion of. While the decahydrate sodium sulfate has been known as Glaubers salt or mirabilis. Another way to dry an ether layer is to wash it with saturated NaCl solution brine before adding drying agent. If you collected a substantial amount of.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Any water dissolved in the ether can be removed by utilizing a drying agent such as anhydrous magnesium sulfate MgSO 4 and filtering off the hydrate MgSO 4 xH 2 O that forms. Put 15 mL of coffee and tea solution in a separatory funnel 3. Sodium SulfateAnhydrous ManufacturerSupplier Trade name. 1266 K anhydrous decomposes Solubility in water. Set a separatory funnel 2.
 Source: mistralni.co.uk
Source: mistralni.co.uk
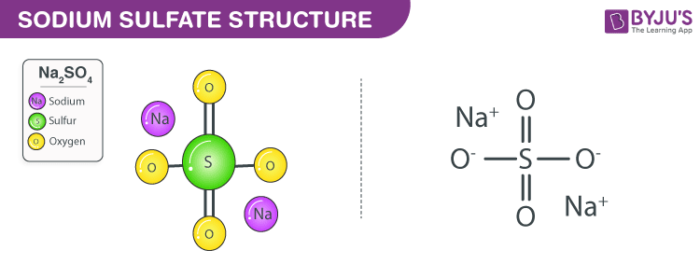
Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent. Identification Product form. Sodium Sulfate Anhydrous CAS-No. Sodium sulfate also known as sodium sulphate or sulfate of soda is the inorganic compound with formula Na 2 SO 4 as well as several related hydratesAll forms are white solids that are highly soluble in water. Sodium citrate is the sodium salt of citric acidIt is white crystalline powder or white granular crystals slightly deliquescent in moist air freely soluble in water practically insoluble in alcoholLike citric acid it has a sour tasteFrom the medical point of view it is used as alkalinizing agent.
 Source: cs.mcgill.ca
Source: cs.mcgill.ca
It is mainly used as a filler in the manufacture of powdered home laundry. Identification Product form. 993 C 1819 F. Set a separatory funnel 2. 1266 K anhydrous decomposes Solubility in water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Swirl the contents of the flask. 436 g100 mL 0 C 45 g100 mL 7 C 529 g100 mL 20 C 105 g100 mL 96 C Solubility. Add 05 g of anhydrous sodium sulfate to the combined dichloromethane extracts in the 25 ml Erlenmeyer flask. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents.

Sodium SulfateAnhydrous Created by Global Safety Management Inc. 58 Monday March 26 2012 Rules and Regulations Date of issue. 993 C 1819 F. The dry ether solution is. While the decahydrate sodium sulfate has been known as Glaubers salt or mirabilis.
 Source: chemtradeasia.com
Source: chemtradeasia.com
Melting point apparatus Procedure. Put 15 mL of coffee and tea solution in a separatory funnel 3. Decomposes to cupric oxide at 650 C Solubility in water. Pentahydrate soluble in methanol 104 gL 18 C insoluble in ethanol. 385 g100 mL methanol 86 g100 mL acetone soluble in ethanol ether pyridine glycerol.
 Source: byjus.com
Source: byjus.com
Any water dissolved in the ether can be removed by utilizing a drying agent such as anhydrous magnesium sulfate MgSO 4 and filtering off the hydrate MgSO 4 xH 2 O that forms. Sodium SulfateAnhydrous ManufacturerSupplier Trade name. SO 2 2NaOH Na 2 SO 3 H 2 O. If you collected a substantial amount of. 11 04112018 EN English US Page 1 SECTION 1.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1266 K anhydrous decomposes Solubility in water. Set a separatory funnel 2. Draw off the aqueous layer. Anhydrous insoluble in ethanol. 15 mL of saturated sodium chloride solution to the separatory funnel to dry the ethyl acetate layer.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title anhydrous sodium sulfate melting and boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.