Anhydrous potassium melting point
Home » datasheet » Anhydrous potassium melting pointAnhydrous potassium melting point
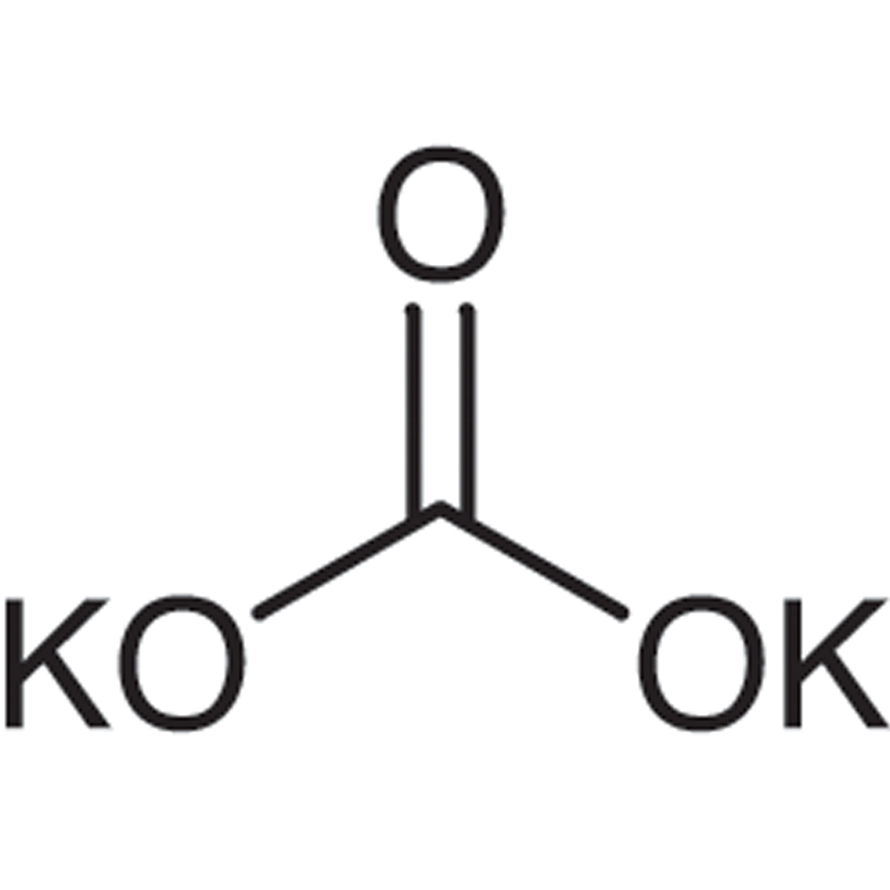
Anhydrous Potassium Melting Point. Ammonia is a toxic gas or liquid that when concentrated is corrosive to tissues upon contact. Preparations of Sodium Sulphate. Potassium carbonate is a potassium salt that is the dipotassium salt of carbonic acid. 1502 C 2736 F.
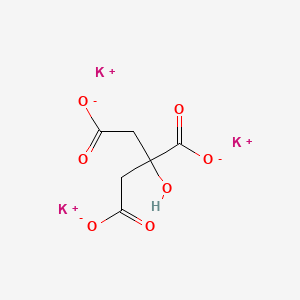
Ammonia is a toxic gas or liquid that when concentrated is corrosive to tissues upon contact. CID 767 Carbonic acid CID 5462222 Potassium Dates. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 1364 F58 C to 13712 F584 C dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room temperature without forming. 2664gmml anhydrous 1464gmml decahydrate Refractive index. 858 C 1576 F. Sort by Relevance.
For the purposes of this card ammonia refers to solutions that are 50 ammonia or greater ammonia anhydrous and ammonia anhydrous liquified unless otherwise specified.
It has a role as a catalyst a fertilizer and a flame. For the purposes of this card ammonia refers to solutions that are 50 ammonia or greater ammonia anhydrous and ammonia anhydrous liquified unless otherwise specified. Soluble in water glycerol and hydrogen iodide and insoluble in ethanol. CID 767 Carbonic acid Component Compounds. Analytical 4 ACS reagent 2 BioReagent 2 Technique. It has a role as a catalyst a fertilizer and a flame.
 Source: cymitquimica.com
Source: cymitquimica.com
Ammonia is a toxic gas or liquid that when concentrated is corrosive to tissues upon contact. Magnetic susceptibility χ 23610 6 cm 3 mol Structure Crystal structure. Ammonia in container may explode in heat of fire. In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the. For the purposes of this card ammonia refers to solutions that are 50 ammonia or greater ammonia anhydrous and ammonia anhydrous liquified unless otherwise specified.
 Source: lobachemie.com
Source: lobachemie.com
Potassium chlorate and sulfuric acid react to cause fire and possible explosions Mellor 2315. Magnetic susceptibility χ 23610 6 cm 3 mol Structure Crystal structure. Potassium carbonate is a potassium salt that is the dipotassium salt of carbonic acid. For the purposes of this card ammonia refers to solutions that are 50 ammonia or greater ammonia anhydrous and ammonia anhydrous liquified unless otherwise specified. CID 767 Carbonic acid Component Compounds.
 Source: scbt.com
Source: scbt.com
Soluble in water glycerol and hydrogen iodide and insoluble in ethanol. Potassium carbonate is a potassium salt that is the dipotassium salt of carbonic acid. Clear colorless gasClear colorless liquid under pressure. Ammonia in container may explode in heat of fire. Keyword 7647-01-0 Showing 1-19 of 19 results for 7647-01-0 within Products.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Magnetic susceptibility χ 23610 6 cm 3 mol Structure Crystal structure. Ammonia in container may explode in heat of fire. In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the. 858 C 1576 F. 2664gmml anhydrous 1464gmml decahydrate Refractive index.
 Source: fishersci.dk
Source: fishersci.dk
884 o C anhydrous 324 0 C decahydrate Density. 858 C 1576 F. 1775 K Solubility in water. CID 767 Carbonic acid CID 5462222 Potassium Dates. 2664gmml anhydrous 1464gmml decahydrate Refractive index.

Sort by Relevance. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311. Mixing of ammonia with several chemicals can cause severe fire hazards andor explosions. 1468 anhydrous 1394 decahydrate Solubility. Ammonia is a toxic gas or liquid that when concentrated is corrosive to tissues upon contact.
 Source: fishersci.se
Source: fishersci.se
For the purposes of this card ammonia refers to solutions that are 50 ammonia or greater ammonia anhydrous and ammonia anhydrous liquified unless otherwise specified. Incompatible with many materials including silver and gold salts halogens alkali metals nitrogen trichloride potassium chlorate chromyl chloride oxygen halides acid vapors azides ethylene oxide picric acid and many other chemicals. It has a role as a catalyst a fertilizer and a flame. 1131 K anhydrous 41 C dihydrate 193 C trihydrate Boiling point. Boiling Point C Feature.
 Source: cymitquimica.com
Source: cymitquimica.com
1502 C 2736 F. 858 C 1576 F. Mixing of ammonia with several chemicals can cause severe fire hazards andor explosions. 1502 C 2736 F. Clear colorless gasClear colorless liquid under pressure.
Source: chemspider.com
1131 K anhydrous 41 C dihydrate 193 C trihydrate Boiling point. Ammonia is a toxic gas or liquid that when concentrated is corrosive to tissues upon contact. Soluble in HF insoluble in alcohol. Clear colorless gasClear colorless liquid under pressure. If a drop of a solution of sulfur dioxide in ether or alcohol is added to powdered potassium chlorate the mass explodes Mellor 2311.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Analytical 4 ACS reagent 2 BioReagent 2 Technique. Boiling Point C Feature. 1775 K Solubility in water. Ammonia in container may explode in heat of fire. 884 o C anhydrous 324 0 C decahydrate Density.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title anhydrous potassium melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.