Aluminum oxide melting point
Home » datasheet » Aluminum oxide melting pointAluminum oxide melting point
Aluminum Oxide Melting Point. The arc from AC welding. ə ˈ lj uː m ɪ n əm. Water gl Organic Solvents. Aluminum oxide Aluminum oxide exists in several different crystallographic forms of which corundum is most common.
 Aluminium Oxide Properties Production And Applications Matmatch From matmatch.com
Aluminium Oxide Properties Production And Applications Matmatch From matmatch.com
The stable oxide layer which protects aluminium from corrosion may be artificially enhanced by anodising In this process the aluminium is connected to a circuit as shown in Fig. Since its near the top of the table its atoms are relatively light compared to elements lower down the table such as lead. Bauxite contains iron and hydrated aluminum oxide with the latter representing its largest. Nitrous oxide refrigerated liquid appears as a colorless liquid. Once the aluminum compound is ready electrolysis is used to extract useful aluminium metal. Aluminum has to be cleaned no matter what process you use but its especially crucial with direct current.
Copperaluminium alloys are to make.
314 where it forms the anode. Aluminum is ductile and has a low melting point and density. Since its near the top of the table its atoms are relatively light compared to elements lower down the table such as lead. Strength at Low Temperatures. Further processing through smelting purifies the aluminum to around 9997. Boils to give a colorless gas that is sweet-smelling and moderately toxic.
 Source: researchgate.net
Source: researchgate.net
For example ice is a solid form of water that melts at 0 degrees Celsius32 degrees Fahrenheit and changes to its liquid form. Bauxite contains iron and hydrated aluminum oxide with the latter representing its largest. In theory the melting point of a solid is the same as the freezing point of the liquid the point at which it turns into a solid. Each pot is made of a steel shell lined with carbon. 2000C 3630F Reaction with Acids.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Acetylglycerrhetinic acid aluminium salt 29728-34-5 156212. The heat generated melts the iron and the aluminum oxide which pour out of the hole in the bottom of the pot. Aluminum can now be produced from clay but the process is not economically feasible at today. Its high melting and boiling points in addition to its excellent thermal resistive properties make aluminium oxide desirable in the manufacture of high-temperature furnace insulations and electrical insulators. Aluminum is in group 13 III of the periodic table which means it loses three electrons to form positive ions it has a valency of 3.
 Source: researchgate.net
Source: researchgate.net
We say that such a body melts. Melting point is the temperature at which a solid turns into a liquid. It is also a temperature at which a solid crystal turns into a liquid. 126 coordination number 3 ppm. 270 at 20 C 68 F valence.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Vapor pressure is at about 745 psig at 70F. The heat generated melts the iron and the aluminum oxide which pour out of the hole in the bottom of the pot. If you try to weld oxidized aluminum the oxide will contaminate the weld and prevent it from solidifying. The stable oxide layer which protects aluminium from corrosion may be artificially enhanced by anodising In this process the aluminium is connected to a circuit as shown in Fig. Aluminum has to be cleaned no matter what process you use but its especially crucial with direct current.
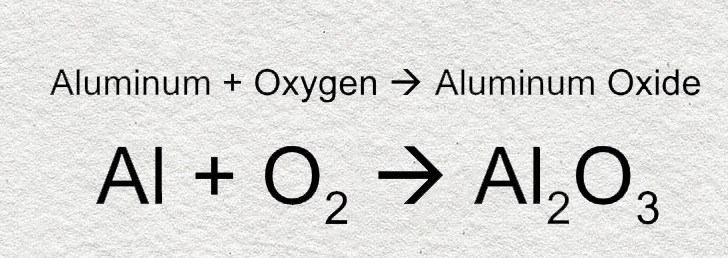 Source: aluminummanufacturers.org
Source: aluminummanufacturers.org
Each pot is made of a steel shell lined with carbon. And upon further weathering as bauxite and iron-rich. The result will be an ugly brittle bead. Although aluminum is the most abundant metal in the earths crust it is never found free in natureAll of the earths aluminum has combined with other elements to form compounds. However aluminum does not occur in its pure metallic form but rather as hydrated aluminum oxide a mixture of water and alumina combined with silica iron oxide and titania.
 Source: sciencedirect.com
Source: sciencedirect.com
The heat generated melts the iron and the aluminum oxide which pour out of the hole in the bottom of the pot. The result will be an ugly brittle bead. 1s 2 2s 2 2p 6 3s 2 3p 1. Once the aluminum compound is ready electrolysis is used to extract useful aluminium metal. While they are liquid they will usually.
 Source: matmatch.com
Source: matmatch.com
The aluminum oxide is almost always combined with other metals to form alloys with. 126 coordination number 3 ppm. 660 C 1220 F boiling point. Its ductility allows aluminum products to be formed close to the end of the products design. The oxide ions can approach the aluminum ion more closely than than they can approach the ferric ion.
 Source: researchgate.net
Source: researchgate.net
Then the crushed ore is mixed with caustic soda and processed in a grinding mill to produce a slurry a watery suspension containing very fine particles. 2467 C 4473 F specific gravity. And the Hall-Heroult process of smelting the aluminum oxide to release pure aluminum. More atomc size properties. If you try to weld oxidized aluminum the oxide will contaminate the weld and prevent it from solidifying.
 Source: aluminummanufacturers.org
Source: aluminummanufacturers.org
93347 K 66032. More atomc size properties. Aluminum oxide Aluminum oxide exists in several different crystallographic forms of which corundum is most common. Van der Waals radius. It has a great affinity towards oxygen and forms a protective layer of oxide on the surface when exposed to air.
 Source: researchgate.net
Source: researchgate.net
Each pot is made of a steel shell lined with carbon. It is classified as a post-transition metal and a poor metal. Melting point is the temperature at which a solid turns into a liquid. Molecular single bond covalent radius. Product Melting Point o CBoiling Point o CAgate.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title aluminum oxide melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.