Alkyl chloride melting point
Home » datasheet » Alkyl chloride melting pointAlkyl chloride melting point
Alkyl Chloride Melting Point. An alkyl group is an alkane-based molecular fragment that bears one open valence for bonding. Hazardous Substances Data Bank HSDB 324 Solubility. Ordinarily the C-C single bond distance is 153 Å. Due to resonance in chlorobenzene C-CI bond is shorter and hence.
 Physical Properties Of Haloalkanes Haloarenes Examples Videos From toppr.com
Physical Properties Of Haloalkanes Haloarenes Examples Videos From toppr.com
Wiley-Interscience Wiley Sons Inc. 288 Shows mechanism SN2 for primary alcohol. A mixture of zinc oxide and hexachloroethane is used in some smoke grenades. Boiling point generally increases with increase in the size of aryl group or halogen atom. The Lucas reagent is a solution of anhydrous zinc chloride and concentrated hydrochloric acid. A similar solubility switch exists for basic organic compounds.
In the presence of iron salts thermal decompositon can occur whicn in some cases can become explosive.
Wiley-Interscience Wiley Sons Inc. Ordinarily the C-C single bond distance is 153 Å. Wiley-Interscience Wiley Sons Inc. Upon ignition these compounds react to form a smoke of zinc chloride which serves as a smokescreen. Many of the carboxylic acids are strong enough that they can be. Crisco by partial hydrogenation of their unsaturated components.
 Source: slideplayer.com
Source: slideplayer.com
No mention of S N i or stereochemistry. Impressively this quite-pure product was isolated with a percent yield of 62 significantly greater than the single-digit yields reported by some other students. To melting-point NMR and TLC analysis. Solomons 8th ed p. Hazardous Substances Data Bank HSDB 324 Solubility.
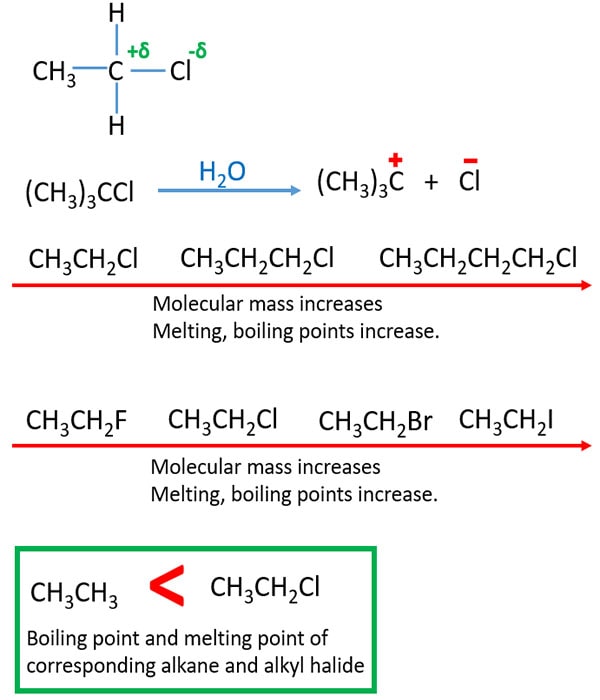 Source: chemistryscl.com
Source: chemistryscl.com
Solomons 8th ed p. A similar solubility switch exists for basic organic compounds. This reagent is very useful in the preparation of alkyl chlorides. No mention of S N i or stereochemistry. At this point the de-protonated organic salt would reside in the aqueous layer.
 Source: toppr.com
Source: toppr.com
Impressively this quite-pure product was isolated with a percent yield of 62 significantly greater than the single-digit yields reported by some other students. While this loss of product was significant it can. Alkyl fluorides are synthesised by heating an alkyl chloridebromide in presence of _____ or _____. Alkyl halides are prepared from alcohols by treating with i HCl ZnCl 2 ii Red P Br 2 iii H 2 SO 4 KI iv All the above 43. Impressively this quite-pure product was isolated with a percent yield of 62 significantly greater than the single-digit yields reported by some other students.

TC 192 BASF Corporation. Solomons 8th ed p. 608 Shows conversion of primary alcohol to primary alkyl chloride SN2 No stereochemistry shown. The nickel chlorides are deliquescent absorbing moisture from the air to form a solution. While this loss of product was significant it can.

The nickel chlorides are deliquescent absorbing moisture from the air to form a solution. McMurry 6th ed p. At this point the de-protonated organic salt would reside in the aqueous layer. 506-507 Shows conversion of primary alcohol to primary alkyl chloride via S N 2. In the presence of iron salts thermal decompositon can occur whicn in some cases can become explosive.
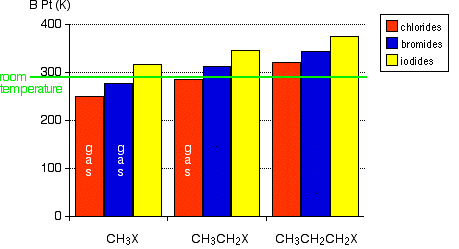 Source: chem.libretexts.org
Source: chem.libretexts.org
A mixture of zinc oxide and hexachloroethane is used in some smoke grenades. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid triglycerides eg. They are generally abbreviated with the symbol for any organyl group R although Alk is sometimes used to specifically symbolize an alkyl group as opposed to an alkenyl group or aryl group. Ed Saxs Dangerous Properties of Industrial Materials. Boiling point order Ar I Ar Br Ar Cl Ar F 3.

Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid triglycerides eg. Due to resonance in chlorobenzene C-CI bond is shorter and hence. National Pesticide Information Retrieval Systems Database on Didecyl Dimethyl Ammonium Chloride 7173-51-5. Wiley-Interscience Wiley Sons Inc. This reagent is very useful in the preparation of alkyl chlorides.

Crisco by partial hydrogenation of their unsaturated components. Some of the remaining. While this loss of product was significant it can. Crisco by partial hydrogenation of their unsaturated components. Wiley-Interscience Wiley Sons Inc.
 Source: slideplayer.com
Source: slideplayer.com
The melting point of p -isomer is more than 0- and m-isomer. Ordinarily the C-C single bond distance is 153 Å. NickelII chloride or just nickel chloride is the chemical compound NiCl 2The anhydrous salt is yellow but the more familiar hydrate NiCl 2 6H 2 O is green. An alkyl group is an alkane-based molecular fragment that bears one open valence for bonding. The Lucas reagent is a solution of anhydrous zinc chloride and concentrated hydrochloric acid.
 Source: pdfprof.com
Source: pdfprof.com
I Ca F 2 ii CoF 2 iii Hg 2 F 2 iv NaF. 1-decanaminium N-decyl-NN-dimethyl- chloride 120. A similar solubility switch exists for basic organic compounds. Ordinarily the C-C single bond distance is 153 Å. Since fats are valued over oils by some Northern European and North American populations vegetable oils are extensively converted to solid triglycerides eg.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title alkyl chloride melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.