Alkyl chloride boiling point
Home » datasheet » Alkyl chloride boiling pointAlkyl chloride boiling point
Alkyl Chloride Boiling Point. C water or benzene. Names of the alkyl substituents on the nitrogen and. Its use as a general purpose disinfectant and pesticide in water treatment of cooling towers and as a wood preservative will result in its direct release to the environment. 608 Shows conversion of primary alcohol to primary alkyl chloride SN2 No stereochemistry shown.

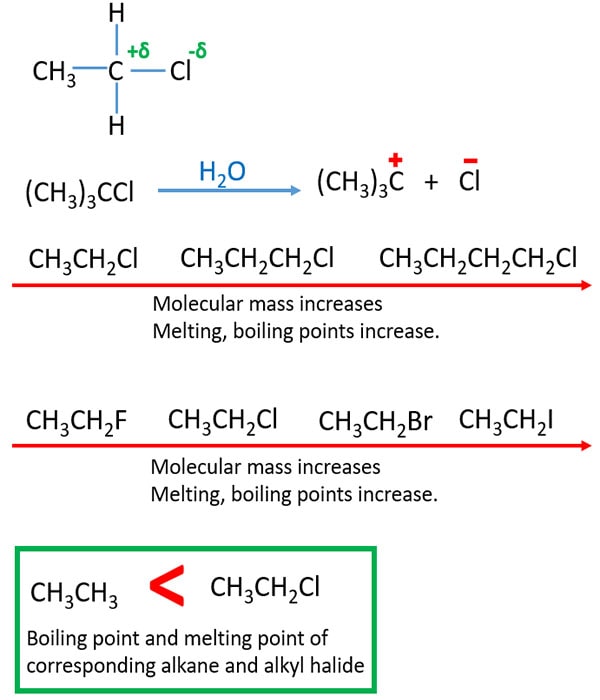
A sharp boiling point is an indication of the purity of the ester. Set up the distillation equipment as. To the mixture in the round bottom flask as prepared above add boiling chips to prevent bumping. Log Octanol-Water Partition Coef SRC. For example compare isobutane 2-methylpropane and n-butane butane which boil at 12 and 0 C and 22-dimethylbutane and 23-dimethylbutane which boil at 50 and 58 C respectively. 608 Shows conversion of primary alcohol to primary alkyl chloride SN2 No stereochemistry shown.
Alkyl fluorides are synthesised by heating an alkyl chloridebromide in presence of _____ or _____.
3526 Adapted Stein Brown method Melting Pt deg C. C water or benzene. No mention of S N i or stereochemistry. 4 - Includes organic compounds with more than one benzene ring and which have a boiling point greater than or equal to 100 ºC. Its use as a general purpose disinfectant and pesticide in water treatment of cooling towers and as a wood preservative will result in its direct release to the environment. 288 Shows mechanism SN2 for primary alcohol.
 Source: toppr.com
Source: toppr.com
I Ca F 2 ii CoF 2 iii Hg 2 F 2 iv NaF. B water or 1-octanol. The Lucas reagent is a solution of anhydrous zinc chloride and concentrated hydrochloric acid. We would like to show you a description here but the site wont allow us. 608 Shows conversion of primary alcohol to primary alkyl chloride SN2 No stereochemistry shown.

293 Mean VP of Antoine Grain methods MP exp database. McMurry 6th ed p. The Lucas reagent is a solution of anhydrous zinc chloride and concentrated hydrochloric acid. Modifications Pollutants removed from the list of hazardous air pollutants. Boiling Pt deg C.

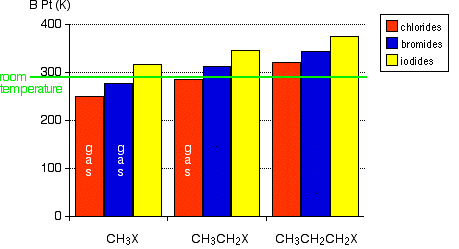
The melting and boiling points of chloro- bromo- and iodoalkanes are higher than the analogous alkanes scaling with the atomic weight and number of halides. The procedure for the distillation of the mixture containing the ester is outlined below. Boiling Pt deg C. B water or 1-octanol. -26 deg C BP exp database.
 Source: chem.libretexts.org
Source: chem.libretexts.org
This effect is due to the increased strength of the intermolecular forcesfrom London dispersion to dipole-dipole interaction because of the increased polarizability. Boiling point and concentrated HCl volume mmoles equivalents Volume mL Mass g mmoles Density Equiv Boiling PointProperties tert-pentyl alcohol 60 48 55 0805 10 Bp102 Concentrated 45 55 12 10 Extremely. To the mixture in the round bottom flask as prepared above add boiling chips to prevent bumping. 293 Mean VP of Antoine Grain methods MP exp database. C water or benzene.

4 - Includes organic compounds with more than one benzene ring and which have a boiling point greater than or equal to 100 ºC. Upon ignition these compounds react to form a smoke of zinc chloride which serves as a smokescreen. I Ca F 2 ii CoF 2 iii Hg 2 F 2 iv NaF. A water or hexane. Its use as a general purpose disinfectant and pesticide in water treatment of cooling towers and as a wood preservative will result in its direct release to the environment.
 Source: slideplayer.com
Source: slideplayer.com
This reagent is very useful in the preparation of alkyl chlorides. 608 Shows conversion of primary alcohol to primary alkyl chloride SN2 No stereochemistry shown. Dichloromethane was separated out during the ethanol boiling stage due to its low boiling point 40 3. If released to air a vapor. 4 - Includes organic compounds with more than one benzene ring and which have a boiling point greater than or equal to 100 ºC.

The boiling point of an alcohol is always significantly higher than that of the analogous alkane. B water or 1-octanol. We shall just be looking at cases where it is replaced by an alkyl group but it could equally well be an aryl group one based on a benzene ring. If this interaction is such that the total energy of the system is lowered then the atoms bond together to form a molecule. The boiling point of an alcohol is always significantly higher than that of the analogous alkane.
 Source: slideplayer.com
Source: slideplayer.com
The procedure for the distillation of the mixture containing the ester is outlined below. 608 Shows conversion of primary alcohol to primary alkyl chloride SN2 No stereochemistry shown. Thus tetraiodomethane CI 4 is a solid whereas tetrachloromethane. Names of the alkyl substituents on the nitrogen and. This reagent is very useful in the preparation of alkyl chlorides.
 Source: slidetodoc.com
Source: slidetodoc.com
No discussion of SN2. Boiling point and concentrated HCl volume mmoles equivalents Volume mL Mass g mmoles Density Equiv Boiling PointProperties tert-pentyl alcohol 60 48 55 0805 10 Bp102 Concentrated 45 55 12 10 Extremely. For example compare isobutane 2-methylpropane and n-butane butane which boil at 12 and 0 C and 22-dimethylbutane and 23-dimethylbutane which boil at 50 and 58 C respectively. -9953 Mean or Weighted MP VPmm Hg25 deg C. -26 deg C BP exp database.
 Source: chemistryscl.com
Source: chemistryscl.com
We shall just be looking at cases where it is replaced by an alkyl group but it could equally well be an aryl group one based on a benzene ring. The dominant factor here is hydrogen bonding and the first table below documents the powerful intermolecular attraction that results from -O-H — O- hydrogen bonding in alcohols light blue columns. In which R and R represent alkyl groups 1 and the functional group is the -CO-O- group. B water or 1-octanol. If this interaction is such that the total energy of the system is lowered then the atoms bond together to form a molecule.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title alkyl chloride boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.