Acetylsalicylic acid melting point
Home » datasheet » Acetylsalicylic acid melting pointAcetylsalicylic acid melting point
Acetylsalicylic Acid Melting Point. The synthesis of aspirin is classified as an esterification reaction. In case of skin contact Wash off with soap and plenty of water. C and compare it to a sample of pure salicylic acid from the stockroom. As you stated the absence of any other spots in the TLC shows that the impure aspirin sample.
 1 Salicylic Acid Content Of Different Samples Of Acetylsalicylic Acid Download Table From researchgate.net
1 Salicylic Acid Content Of Different Samples Of Acetylsalicylic Acid Download Table From researchgate.net
The synthesis of aspirin is classified as an esterification reaction. The melting point of pure acetylsalicylic acid is 135 C 1. A useful synthesis of acetylsalicylic acid was developed in 1893 patented in 1899 marketed under the trade name of aspirin by the Bayer Company in Germany. Dry an Erlenmeyer flask and add 3 grams of salicylic acid to it. 144 - 145 - 355. Mix the solution and keep the flask in warm water for 15 minutes.
144 - 145 - 355.
How were you able to tell the crude aspirin from the. The risk or severity of adverse effects can be increased when Acetylsalicylic acid is combined with Ursodeoxycholic acid. H 2 SO 4 use a dropper H 2 SO 4 is highly corrosive and swirl the flask gently until the salicylic acid dissolves. 4318 K Boiling point. Aspirin or acetylsalicylic acid is perhaps the most commonly used analgesic and antipyretic medication worldwide having been in clinical use for over 100 years. Comment on the purity of your aspirin based on its.
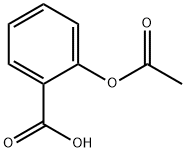 Source: chemicalbook.com
Source: chemicalbook.com
Tryptophan is the least plentiful of all 22 amino acids and an essential amino acid in humans provided by food Tryptophan is found in most proteins and a precursor of serotoninTryptophan is converted to 5-hydroxy-tryptophan converted in turn to serotonin a neurotransmitter essential in regulating appetite sleep mood and painTryptophan is a natural sedative and present in dairy. Acetylsalicylic Acid 2112 Aspirin 135-136 Maleic Acid 2112 Manufacture of resins 137-139 Benzilic Acid 2121 A carboxylic acid 150-153 Adipic Acid 1202 Used to manufacture nylon 152-153 Citric Acid 1123 Sour taste of citrus fruits 153-154 Mannitol 0201 Manufacture of radio condensers 167-170 Tartaric Acid 1111 In soft drinks cream of tartar 168-170 Itaconic Acid 0112 A dicarboxylic acid 166. Tryptophan is the least plentiful of all 22 amino acids and an essential amino acid in humans provided by food Tryptophan is found in most proteins and a precursor of serotoninTryptophan is converted to 5-hydroxy-tryptophan converted in turn to serotonin a neurotransmitter essential in regulating appetite sleep mood and painTryptophan is a natural sedative and present in dairy. Soon the drug became famous and Bayer a drug and dye firm started producing it at large scale. If inhaled If breathed in move person into fresh air.
 Source: researchgate.net
Source: researchgate.net
SYNTHESIS OF ASPIRIN acetylsalicylic acid Place 20 g 0015 mole of salicylic acid in a 125-mL Erlenmeyer flask. Acetylsalicylic acid is an acetic acid ester derivative of salicylic acid. What is the melting point of pure aspirin. The active ingredient of. Felix Hoffmann a German chemist produced a stable form of acetylsalicylic acid more commonly known as aspirin.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
The conjugate base is a salt and is water soluble. Aspirin can cause several forms of liver injury. Aspirin is a salicylate drug often used as an analgesic to relieve minor aches and pains as an anti-inflammatory compound that inhibits Cox-1Target. How were you able to tell the crude aspirin from the. Bismuth subsalicylate a salt of bismuth and salicylic acid is the active ingredient in stomach-relief aids such as Pepto-Bismol is the main.
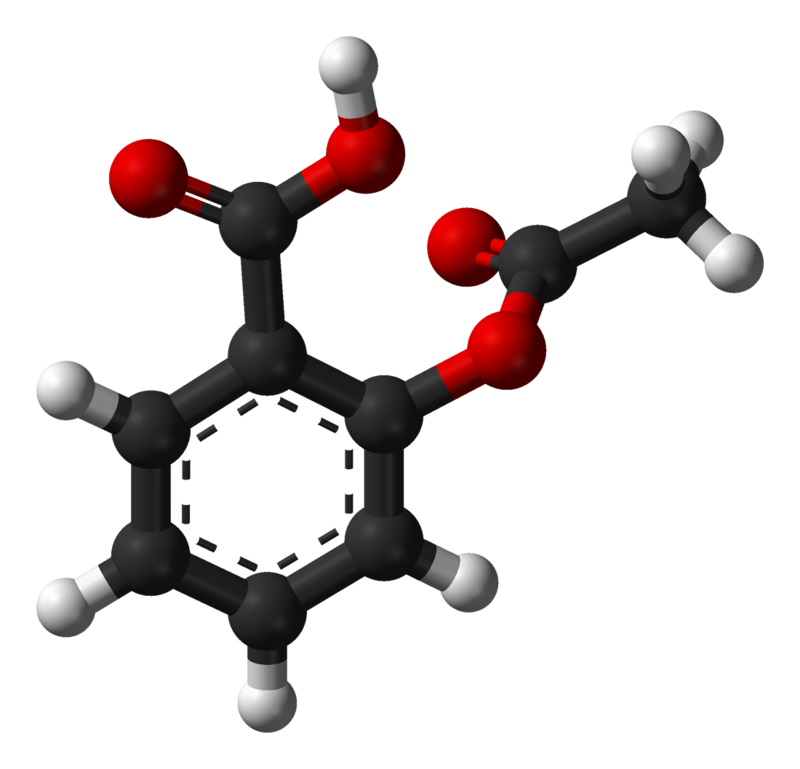 Source: en.wikipedia.org
Source: en.wikipedia.org
Determine the melting point of your purified salicylic acid mp. How were you able to tell the crude aspirin from the. 2-butynedioic acid acetylenedicarboxylic acid HOOCCCCOOH. PERKIN-ELMER 21 GRATING Instrument parameters. The melting point should be recorded as a range the first reading is the temperature at which the sample starts to liquefy and the second reading is taken when the sample is completely melted.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Determine the melting point of your purified salicylic acid mp. The name aspirin was invented by the chemist Felix Hofmann who originally synthesized acetylsalicylic acid for Bayer. The active ingredient of. Aspirin Purity Testing 5. SOLID KBr DISC VS KBr.

Soon the drug became famous and Bayer a drug and dye firm started producing it at large scale. What color does iron III chloride turn a solution of aspirin if it contains traces of salicylic acid. The active ingredient of. The name aspirin was invented by the chemist Felix Hofmann who originally synthesized acetylsalicylic acid for Bayer. Comment on the purity of your aspirin based on its.

The salting and melting point of Acetylsalicylic acid is 211 C and 315 C respectively The brightest point of Acetylsalicylic acid is 157 C The concentration of Acetylsalicylic acid is 144 to 20 C Its vapor pressure is 82 x 10-5 mm Hg at 25 C Its LogP is 226 It is the practice of color-correction when exposed to direct sunlight due to its photochemical. It was Bayers brand name for the drug. 1586 C 3175 F. Put 5 to 8 drops of 85 phosphoric acid along with 6 mL of acetic anhydride to the flask. Determine the melting point of your purified salicylic acid mp.
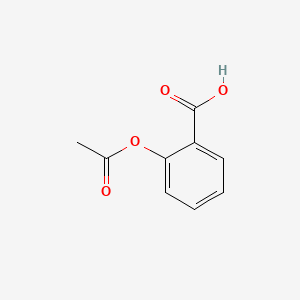
191 - 194 - 37 E-1-propene-123-tricarboxylic acid trans-aconitic acid HOOCCHCCH 2 COOH. It was thin short white crystals and had a melting point range or 154-155 degrees Celsius. In Part I of this. 57 - 218 - 221. C and compare it to a sample of pure salicylic acid from the stockroom.
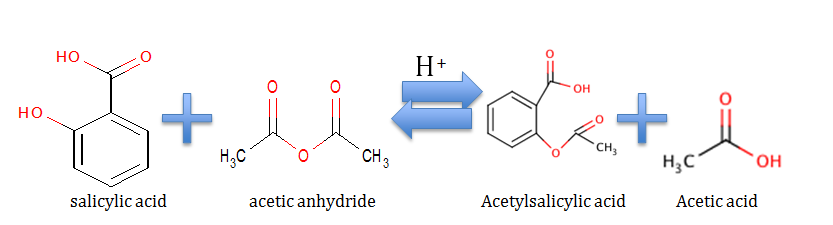 Source: odinity.com
Source: odinity.com
H 2 SO 4 use a dropper H 2 SO 4 is highly corrosive and swirl the flask gently until the salicylic acid dissolves. 4-acetylbenzoic acid—-CH 3 COC 6 H 4 COOH. What is the melting point of pure aspirin. 144 - 145 - 355. Aspirin is a trade name for acetylsalicylic acid a common analgesic.
 Source: researchgate.net
Source: researchgate.net
191 - 194 - 37 E-1-propene-123-tricarboxylic acid trans-aconitic acid HOOCCHCCH 2 COOH. Aspirin or acetylsalicylic acid is perhaps the most commonly used analgesic and antipyretic medication worldwide having been in clinical use for over 100 years. Acetylsalicylic Acid 2112 Aspirin 135-136 Maleic Acid 2112 Manufacture of resins 137-139 Benzilic Acid 2121 A carboxylic acid 150-153 Adipic Acid 1202 Used to manufacture nylon 152-153 Citric Acid 1123 Sour taste of citrus fruits 153-154 Mannitol 0201 Manufacture of radio condensers 167-170 Tartaric Acid 1111 In soft drinks cream of tartar 168-170 Itaconic Acid 0112 A dicarboxylic acid 166. The produced PhO 2 FeII species is a dark purple color the presence of which indicates the presence of. The melting point should be recorded as a range the first reading is the temperature at which the sample starts to liquefy and the second reading is taken when the sample is completely melted.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title acetylsalicylic acid melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.