Acetone boiling point at 08 atm
Home » datasheet » Acetone boiling point at 08 atmAcetone boiling point at 08 atm
Acetone Boiling Point At 08 Atm. Liquid Densities - Densities of common liquids like acetone beer oil water and more. Cinnamaldehyde 13216 gmol is used as a flavoring agent. For ethanol Kb 122Cm. 56 C LabNetwork old LN00163394.
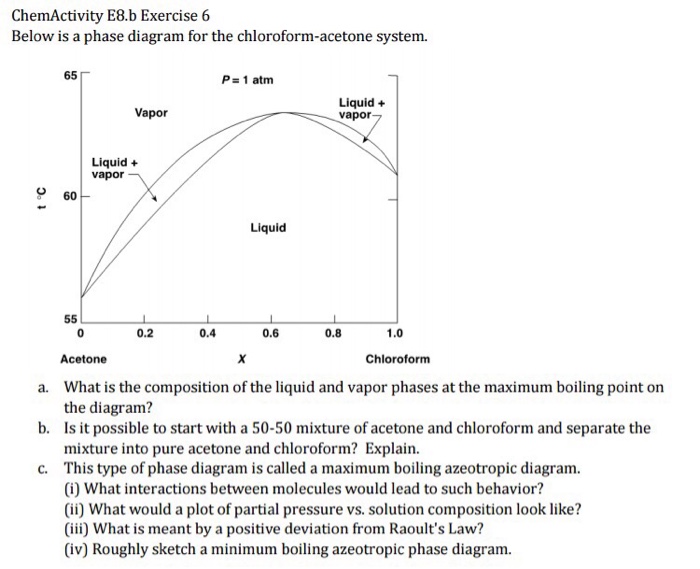
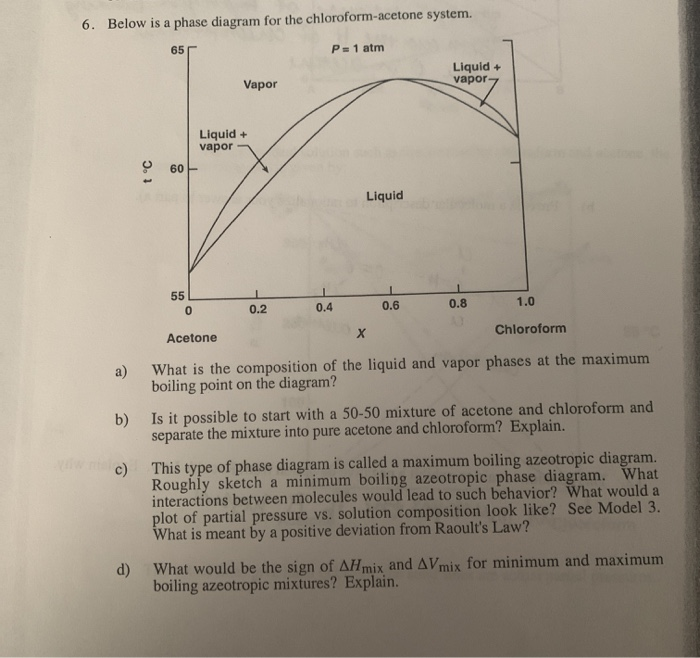
Then the hands do not give the keys some unknown acceleration during the motion we consider. Cinnamaldehyde 13216 gmol is used as a flavoring agent. However the distillation can be a long process and the water may damage some other compounds. Acetone is a manufactured chemical that is also found naturally in the environment. 33430 K decomposes at 450 C Solubility in water. Boiling-point Txy diagram shown in Figure 41-1.
Determine the temperature at which its pressure will drop at sea level A.
It has been isolated from Achnatherum robustum. The water safeguards some components by preventing overheating as the temperature will not exceed 100 C the boiling point of water at normal pressure. Q30 If density of final solution is 101 gm ml then find the molarity of the ion in resulting solution by which nature of the above solution is identified is A 05 M B 08 M C 04 M D 1. 33430 K decomposes at 450 C Solubility in water. The initial point is really just after the keys leave the f irst students hand and the f inal point is just before the second woman catches them. 56 C LabNetwork old LN00163394.
 Source: ddbst.com
Source: ddbst.com
Solvent Freezing point C Kf Ckgmole acetone -954 24 0 benzene 55 512 cyclohexane 65 201 water 00 186 Notice that the freezing point of a substance or a mixture is the temperature at which the solid and liquid phases are in equilibrium at one atm of pressure. The initial point is really just after the keys leave the f irst students hand and the f inal point is just before the second woman catches them. Acetone is used to make plastic fibers drugs and other chemicals. Pure fluids available in the REFPROP 10 program including most fixed properties such as the critical point normal boiling point heating value T and P limits of the equation of state and so on. A well-known example of a positive azeotrope is 9563 ethanol and 437 water by mass which boils at 782 C.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
1 mol of liquid ethanol boils at on Lv 854times 105 Jkg th. Liquid Densities - Densities of common liquids like acetone beer oil water and more. Inputs Format required to calculate properties for mixtures including the. 56 C Alfa Aesar. Cinnamaldehyde 13216 gmol is used as a flavoring agent.
 Source: researchgate.net
Source: researchgate.net
For ethanol Kb 122Cm. It evaporates easily is flammable and dissolves in water. Q30 If density of final solution is 101 gm ml then find the molarity of the ion in resulting solution by which nature of the above solution is identified is A 05 M B 08 M C 04 M D 1. Solvent Freezing point C Kf Ckgmole acetone -954 24 0 benzene 55 512 cyclohexane 65 201 water 00 186 Notice that the freezing point of a substance or a mixture is the temperature at which the solid and liquid phases are in equilibrium at one atm of pressure. 1 NTP - Normal Temperature and Pressure - is defined as 20 o C 29315 K 68 o F and 1 atm 101325 kNm2 101325 kPa 147 psia 0 psig 30 in Hg 760 torr Since specific gravity is the ratio between the density mass per unit volume of an actual gas and the density of air - specific gravity has no dimension.

For ethanol Kb 122Cm. It is a colorless liquid with a distinct smell and taste. The region between these two curve is the two-phase region. 133 F 760 mmHg. The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents a positive azeotrope or greater than the boiling point of any of its constituents a negative azeotrope.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
56 C Alfa Aesar 22928 32451 43053 19392 30698 L10407. A key point to remember is that in python arrayvector indices start at 0. What mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 8257C. Calculation of density of acetone in kgm3 and estimate the density of a mixture of 50 acetone and 50 water by volume additivity. The density of air at NTP is.

The region between these two curve is the two-phase region. The acceleration between these points is the known acceleration caused by the planets gravitation. Unlike Matlab which uses parentheses to index a array we use brackets in python. 100 gL 19 C Vapor pressure. In Figure 41-1 if we start with a cold liquid mixture of x1 0217 and heat the mixture it will start to boil at 400 K and the composition.
 Source: chegg.com
Source: chegg.com
Henrys law constants at 25 C of the studied PFRs vary between 28 10 4 atm-m 3. 562 C FooDB FDB008301. 56 C Alfa Aesar. It has been isolated from Achnatherum robustum. Ethanol boils at 784 C water boils at 100 C but the.

100 gL 19 C Solubility in dimethyl sulfoxide. A well-known example of a positive azeotrope is 9563 ethanol and 437 water by mass which boils at 782 C. Solucionario quimica de raymond chang 12 edicion. Academiaedu is a platform for academics to share research papers. It is also called dimethyl ketone 2-propanone and beta-ketopropane.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Free essays homework help flashcards research papers book reports term papers history science politics. Calculation of density of acetone in kgm3 and estimate the density of a mixture of 50 acetone and 50 water by volume additivity. 56 C Alfa Aesar. The next exercise illustrates how to use the experimentally measured decrease of freezing point Tf to calculate the. 56 C Food and Agriculture Organization of the United Nations Propan-2-one.
 Source: researchgate.net
Source: researchgate.net
We can select a range of elements too. What mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 8257C. It has been isolated from Achnatherum robustum. For ethanol Kb 122Cm. Inputs Format required to calculate properties for mixtures including the.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title acetone boiling point at 08 atm by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.