Acetic anhydride boiling point
Home » datasheet » Acetic anhydride boiling pointAcetic anhydride boiling point
Acetic Anhydride Boiling Point. Special Warnings from DuPont. The 50 mL Erlenmeyer flask with the mixture of salicylic acid acetic anhydride and phosphoric acid was partially submerged in. 117 mm2s 20 C Solubility. Boiling point of water.
 Acetic Anhydride From yumpu.com
Acetic Anhydride From yumpu.com
Williamsons synthesis of preparing dimethyl ether is. The fabric may or may not offer barrier. Boiling point of water. Acetone CH 3 COCH 3. Potassium hydroxide residue in a catalyst pot reacted violently when acetic acid was added MCA Case History 920. 100 C 212 F Boiling point of water in Kelvin.
Acetic Anhydride ManufacturerSupplier Trade name.
S25119A Recommended uses of the product and restrictions on use. S25119A Recommended uses of the product and restrictions on use. The 50 mL Erlenmeyer flask with the mixture of salicylic acid acetic anhydride and phosphoric acid was partially submerged in. How many grams of salicylic acid are needed to make 1000 1-gram tablets of aspirin. 100 C 212 F Boiling point of water in Kelvin. DIN 53171 Flash point 120 F 49.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The formula for this reaction is C 7 H 6 O 3 C 4 H 6 O 3 C 9 H 8 O 4 HC 2 H 3 O 2. Aspirin is prepared from the reaction of salicylic acid C 7 H 6 O 3 and acetic anhydride C 4 H 6 O 3 to produce aspirin C 9 H 8 O 4 and acetic acid HC 2 H 3 O 2. Moderately toxic and an irritant. A Formation of pentaacetate of glucose with acetic anhydride. A blank cell indicates the fabric has not been tested.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A Formation of pentaacetate of glucose with acetic anhydride. Williamsons synthesis of preparing dimethyl ether is. The carboxyl functional group in ethanoic acid can cause ionization of the compound given by. Solids prepared in this manner serve a derivative whose. Addition of a small amount of water.
 Source: chemsynthesis.com
Source: chemsynthesis.com
A blank cell indicates the fabric has not been tested. A 70-80 C hot water bath was prepared by placing a 250 mL beaker on a hot plate with a thermometer to monitor temperature. 20 grams of salicylic acid 50 mL of acetic anhydride and 5 drops of 85 phosphoric acid solution were placed into a 50 mL Erlenmeyer flask. LD 50 oral. Assume 100 percent yield Solution.
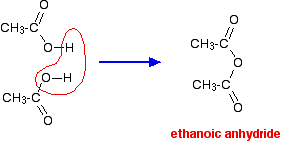 Source: chemguide.co.uk
Source: chemguide.co.uk
The carboxyl functional group in ethanoic acid can cause ionization of the compound given by. The product of the condensation of two molecules of acetic acid is acetic anhydride. Addition of a small amount of water. The main target users are workers and those responsible for occupational safety and health. 3732 K Boiling point of ethanol.
 Source: yumpu.com
Source: yumpu.com
Succinic anhydride appears as colorless needles or white crystalline solid. Assume 100 percent yield Solution. DIN 53171 Flash point 120 F 49. 7837 C 1731 F Boiling point of methanol. A Formation of pentaacetate of glucose with acetic anhydride.

Pure acetic acid often called glacial acetic acid is a corrosive colourless liquid boiling point 1179 C 2442 F. Potassium hydroxide residue in a catalyst pot reacted violently when acetic acid was added MCA Case History 920. The fabric may or may not offer barrier. Suffice it to say that since the sulfuric acid that is used during in the reaction is re-produced at the end of the reaction mechanism sulfuric acid is acting as a catalyst for the reaction. Succinic anhydride appears as colorless needles or white crystalline solid.
-269 C -452 F. 116 - 118 C 1013 hPa Density. The fabric may or may not offer barrier. Aspirin an acetyl derivative of. Special Warnings from DuPont.
 Source: studylib.net
Source: studylib.net
The main process involves dehydration of acetic acid to give ketene at 700750 C. S25119A Recommended uses of the product and restrictions on use. The main process involves dehydration of acetic acid to give ketene at 700750 C. The product of the condensation of two molecules of acetic acid is acetic anhydride. And at 198F and 1 mm Hg pressure.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Acetic Anhydride ManufacturerSupplier Trade name. A Formation of pentaacetate of glucose with acetic anhydride. The product of the condensation of two molecules of acetic acid is acetic anhydride. The carboxyl functional group in ethanoic acid can cause ionization of the compound given by. Succinic anhydride appears as colorless needles or white crystalline solid.
 Source: sciencedirect.com
Source: sciencedirect.com
And at 198F and 1 mm Hg pressure. Addition of a small amount of water. Suffice it to say that since the sulfuric acid that is used during in the reaction is re-produced at the end of the reaction mechanism sulfuric acid is acting as a catalyst for the reaction. Melting point 166 C 619 F that is completely miscible with water. The 50 mL Erlenmeyer flask with the mixture of salicylic acid acetic anhydride and phosphoric acid was partially submerged in.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title acetic anhydride boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.