87 gas melting point
Home » datasheet » 87 gas melting point87 gas melting point
87 Gas Melting Point. To β 87Sr with a total disintegration energy of 0283 MeV 2 the only stable isotope is 85Rb but has an extremely slow decay rate thus making it effectively stable. Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions Related Documents Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each molecule are given for 150 different alcohols and acids. There is no question that methane is doing some very odd and worrying things said Euan Nisbet an atmospheric scientist at Royal Holloway University of London. 87 - Ionization energy.
 Rubidium Chemical Element Britannica From britannica.com
Rubidium Chemical Element Britannica From britannica.com
Hydrogen exists in two different spin isomers of hydrogen diatomic molecules that differ by the. Its covalent radius is 315 pm. Melting Point ºC Boiling Point ºC Density gcm 3 at 293 K 1. At normal atmospheric pressure carbon does not melt when heated it sublimes. It has two distinct oxidation states 1 -1 which make it able to act as both an oxidizing and a reducing agent. This is the diagonal line at stage I on the graph.
For chemistry students and teachers.
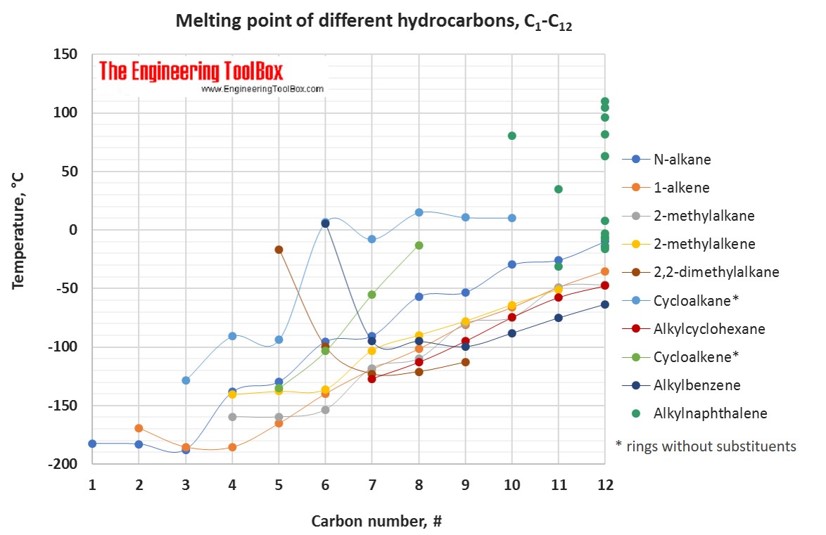
Melting Point ºC Boiling Point ºC Density gcm 3 at 293 K 1. The big question is why. Chemical Properties Handbook. It has two distinct oxidation states 1 -1 which make it able to act as both an oxidizing and a reducing agent. Hydrogen has a melting point of -25914 C and a boiling point of -25287 C. Multisubstituted alkane singelsubstituted alkane singelsubstituted alkene normal alkene normal alkane alkyl cyclohexane alkylbenzene cycloalkene.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The chemical element with the lowest melting point is Helium and the element with the highest melting point is Carbon. This is the diagonal line at stage I on the graph. Hydrogen has a melting point of -25914 C and a boiling point of -25287 C. Hydrogen exists in two different spin isomers of hydrogen diatomic molecules that differ by the. Under a pressure of 28 atmospheres.
 Source: en.citizendium.org
Source: en.citizendium.org
User Defined Compressibility. For hydrocarbons with the same carbon number the boiling point increases in the following order. The mass is taken from the high-precision measurement of 3 and the density melting point boiling point and heat capacities for the naturally occurring form. User Defined Compressibility. We would like to show you a description here but the site wont allow us.
Source: chem.uiuc.edu
There is no question that methane is doing some very odd and worrying things said Euan Nisbet an atmospheric scientist at Royal Holloway University of London. The mass is taken from the high-precision measurement of 3 and the density melting point boiling point and heat capacities for the naturally occurring form. At normal atmospheric pressure arsenic does not melt when heated it sublimes. The big question is why. Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions Related Documents Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each molecule are given for 150 different alcohols and acids.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Over 12000 phrases and expressions. The big question is why. This is the only isotope we consider in this reference. Here we introduce the term tipping element to describe large-scale components of the Earth system that may pass a tipping point. To β 87Sr with a total disintegration energy of 0283 MeV 2 the only stable isotope is 85Rb but has an extremely slow decay rate thus making it effectively stable.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Boiling point C 26893 24608 1858 15322 108 617 density at 0 C 1 atmosphere grams per litre 017847 0899 1784 375 5881 973 solubility in water at 20 C cubic centimetres of gas per 1000 grams water 861 105. 87 - Ionization energy. The mass is taken from the high-precision measurement of 3 and the density melting point boiling point and heat capacities for the naturally occurring form. Hydrogen has a melting point of -25914 C and a boiling point of -25287 C. The largest dictionary of idioms and phrases currently in use in British American and Australian English.
 Source: en.citizendium.org
Source: en.citizendium.org
Adding a heat will convert the solid into a liquid with no temperature change. At normal atmospheric pressure arsenic does not melt when heated it sublimes. In the following table the use row is the value recommended for use in other Wikipedia pages in order to maintain consistency across content. However this highlights one of the challenges with such an alloying approach within SLM as using a higher ED can encourage. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
 Source: thoughtco.com
Source: thoughtco.com
User Defined Compressibility. Over 12000 phrases and expressions. Adding a heat will convert the solid into a liquid with no temperature change. Hydrogen exists in two different spin isomers of hydrogen diatomic molecules that differ by the. Below the melting point the solid is the more stable state of the two.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Under a pressure of 28 atmospheres. Others point to the natural gas fracking boom in North America and its sometimes leaky infrastructure. The mass is taken from the high-precision measurement of 3 and the density melting point boiling point and heat capacities for the naturally occurring form. Z Factor at critical point 304 K 74 MPa 0468. If the pressure is increased to 10 atmospheres carbon graphite is observed to melt at 3550 C.
 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Hydrogen exists in two different spin isomers of hydrogen diatomic molecules that differ by the. Scientists wonder if they will have. Z Factor at critical point 304 K 74 MPa 0468. Once a substance hits its melting point it is a. Hydrogen has a density of 008988 gL making it less dense than air.
 Source: britannica.com
Source: britannica.com
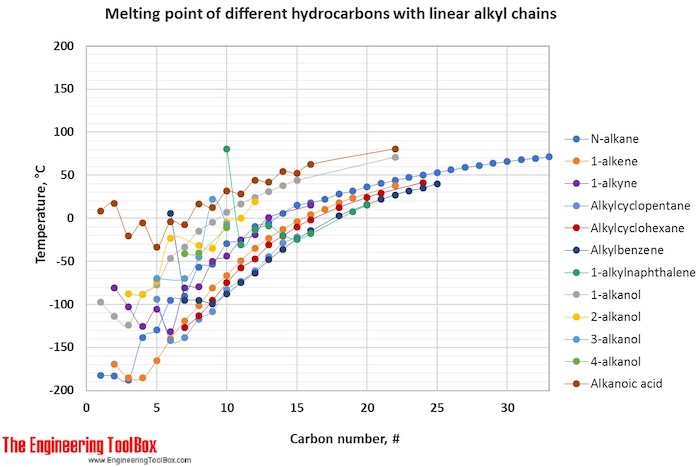
For hydrocarbons with the same carbon number the boiling point increases in the following order. Melting Point ºC Boiling Point ºC Density gcm 3 at 293 K 1. Melting points - freezing points - Documents giving melting or freezing point of elements and different kind of chemical species at varying conditions Related Documents Alcohols and carboxylic acids - physical data - Molweight melting and boiling point density pKa-values as well as number of carbon and hydrogen atoms in each molecule are given for 150 different alcohols and acids. User Defined Compressibility. In the following table the use row is the value recommended for use in other Wikipedia pages in order to maintain consistency across content.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title 87 gas melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.