2 methylpentane boiling point
Home » datasheet » 2 methylpentane boiling point2 methylpentane boiling point
2 Methylpentane Boiling Point. A straight-chain alkane will have a boiling point higher than a branched-chain alkane due to the greater surface area in contact thus the greater van der Waals forces between adjacent molecules. CH 4-1825 -164. Better stacking higher melting point. In dehydrohalogenation a haloalkane is exposed to a base the base then helps the elimination of the halogen and a hydrogen atom.
 2 Methylpentane 107 83 5 Tokyo Chemical Industry Uk Ltd From tcichemicals.com
2 Methylpentane 107 83 5 Tokyo Chemical Industry Uk Ltd From tcichemicals.com
9-16-29-33-61-62 Alfa Aesar L03133. CH 4-1825 -164. K W 45579 M. Specific Heat Helmholtz Energy Gibbs Free Energy Joule-Thomson Coefficient Thermal Conductivity Surface Tension Standard Boiling Point Triple Point Critical Temperature Critical Pressure Acentric Factor Dipole Moment NIST Saturated Vapor Pressure. Acetone CH 3 COCH 3. 9 2001 lists acetone methanol ethanol and.
K W 45579 M.
Citation needed The normal boiling point is just a few. A double bond is formed alkane to alkene. 4 Spectral Information Expand this section. Exercise 1431 When the laboratory reaction described above is run to completion a viscous goop is usually left over in the distillation flask which hardens upon cooling. 9 2001 lists acetone methanol ethanol and. Coefficents calculated by NIST from authors data.
Source: quora.com
Enter the email address you signed up with and well email you a reset link. FLAMMABLE irritates skin and eyes Alfa Aesar L03133 22. B Pentane has a higher boiling point than 2-methylpentane. Based on your knowledge of vapor pressure trends in alkanes predict which compound has the higher boiling point hexane or 2-methylpentane isohexane. Separation of cyclohexene boiling point 83circ from cyclohexanol boiling point 161circ is simple because of the large difference in boiling points between the two liquids.
 Source: patents.google.com
Source: patents.google.com
Separation of cyclohexene boiling point 83circ from cyclohexanol boiling point 161circ is simple because of the large difference in boiling points between the two liquids. Acetic acid anhydride CH 3 COO 2 O. The reason why both fractions predominate in the high boiling component is due to the Evelyn Effect. K W 45579 M. Predicting Boiling Points Arrange the following compounds in order of increasing boiling point.
Source: quora.com
Enter the email address you signed up with and well email you a reset link. Acetic acid anhydride CH 3 COO 2 O. 9 2001 lists acetone methanol ethanol and. 4 Spectral Information Expand this section. 9-16-29-33-61-62 Alfa Aesar L03133.
Source: quora.com
3 Chemical and Physical Properties Expand this section. Isopentane is an extremely volatile and extremely flammable liquid at room temperature and pressureIt is also the least dense liquid at standard conditions. Which of the following statements concerning the boiling points of specific alkanes is correct. A double bond is formed alkane to alkene. Isopentane also called methylbutane or 2-methylbutane is a branched-chain saturated hydrocarbon an alkane with five carbon atoms with formula C 5 H 12 or CHCH 3 2 C 2 H 5.
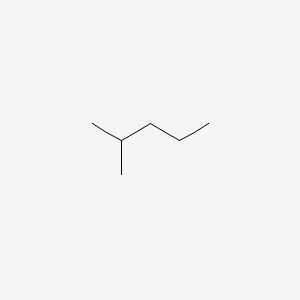
For two alkyl groups on the same carbon its locant is repeated twice 2 4 7-locant set. For two alkyl groups on the same carbon its locant is repeated twice 2 4 7-locant set. Specific Heat Helmholtz Energy Gibbs Free Energy Joule-Thomson Coefficient Thermal Conductivity Surface Tension Standard Boiling Point Triple Point Critical Temperature Critical Pressure Acentric Factor Dipole Moment NIST Saturated Vapor Pressure. 5 g per dL 85 to 95 g per L Percent yield is the ratio of the actual yield to the theoretical yield. For these cases Whitson correlated the Watson Characterization Factor with the more commonly known molecular weight.
 Source: youtube.com
Source: youtube.com
1 Structures Expand this section. Look at these three examples. Based on your knowledge of vapor pressure trends in alkanes predict which compound has the higher boiling point hexane or 2-methylpentane isohexane. Acetone CH 3 COCH 3. CH3-C-CH33 C5H12.
Source: quora.com
Do NOT let this chemical enter the environment. The Saturated Hydrocarbons or Alkanes. Separation of cyclohexene boiling point 83circ from cyclohexanol boiling point 161circ is simple because of the large difference in boiling points between the two liquids. Predicting Boiling Points Arrange the following compounds in order of increasing boiling point. Danger Alfa Aesar L03133.
 Source: tcichemicals.com
Source: tcichemicals.com
Melting Point o C Boiling Point o C State at 25 o C. Better stacking higher melting point. Based on your knowledge of vapor pressure trends in alkanes predict which compound has the higher boiling point hexane or 2-methylpentane isohexane. Do NOT wash away into sewer. A straight-chain alkane will have a boiling point higher than a branched-chain alkane due to the greater surface area in contact thus the greater van der Waals forces between adjacent molecules.
 Source: en.wikipedia.org
Source: en.wikipedia.org
3 Chemical and Physical Properties Expand this section. Alcohol - ethyl grain ethanol C. 2 Names and Identifiers Expand this section. Hence when you compare hexane to its structural isomer 2-methylpentane hexane has a much higher melting point due to the regular arrangement of its structure. FLAMMABLE irritates skin and eyes Alfa Aesar L03133 22.
 Source: fishersci.se
Source: fishersci.se
1 Structures Expand this section. K W 45579 M. Danger Alfa Aesar L03133. 2 Names and Identifiers Expand this section. The midweight alkanes are liquids.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title 2 methylpentane boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.