2 butanol boiling point
Home » datasheet » 2 butanol boiling point2 butanol boiling point
2 Butanol Boiling Point. Jun 22 2019 Correct answer to the question a student in SCH4U titrates a 10. The vapor pressure of a solution containing 13 grams of a nonvolatile solute in 100 grams of water at 25C is 27371 mmHg. Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mlkg of beverages containing orange juice 15 or 40 ethanol and 1 gl of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol. Increasing boiling point and increasing solubility in water.
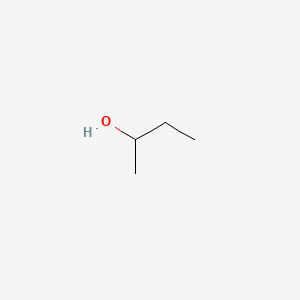
20180 to 20200 C. The vapor pressure of water at 96C is 707 mmHg. Determine the mass KClO3 required to form a saturated solution in 200 g of water at 30 C. In this experiment you will carry out several qualitative tests that will allow you to identify functional groups in organic molecules. It is also known as isopentyl alcohol isopentanol or in the IUPAC recommended nomenclature 3-methyl-butan-1-olAn obsolete name for it was isobutyl carbinol. Calculate the weight of naphthalene in the distillate.
In this experiment you will carry out several qualitative tests that will allow you to identify functional groups in organic molecules.
I Reimer-Tiemann Reaction ii Williamson Synthesis. For simple amino acids such as alanine the pI is an. 5 D x 32 3. In the table below pK a1 and pK a2 for water solutions at 25C are given together with boiling and melting point density and molecular weight as well as number of carbon hydrogen and oxygen atoms in each molecule. 102 deg C 760 mmHg FreezingMelting Point-12 deg C Decomposition TemperatureNot available. 3-chloro-2-butanol affords another example of a compound having two dissimilar chiral carbon atoms and can exist in four stereoisomeric forms as given below.
 Source: pediaa.com
Source: pediaa.com
A diagram to study oxidationreduction reactions. Temperature K A B C Reference Comment. Below the table figures showing the fractions of the different acid forms in aqueous solutions at varying pH are given for some common diprotic organic acids values calculated. VG Nicotine Salts Base. What are the strongest types of intermolecular forces that must be overcome in order to.
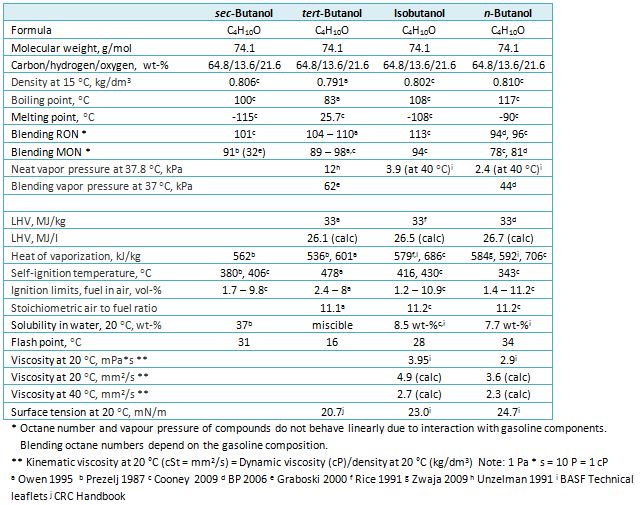 Source: iea-amf.org
Source: iea-amf.org
12100 to 12200 C. Isoamyl alcohol is a colorless liquid with the formula C 5 H 12 O specifically H 3 C 2 CHCH 2 CH 2 OH. Physical state and properties melting point boiling point solubility odor color etc elemental analysis and confirmatory tests for functional groups. Personal Protective Equipment Eyes. It may be seen that structures 1 and 3 1 and 4 2 and 3 2 and 4 represent pairs of diastereomers.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The vapor pressure of water at 96C is 707 mmHg. Determine the mass PbNO32 required to form a saturated solution in 200. Isoamyl alcohol is a colorless liquid with the formula C 5 H 12 O specifically H 3 C 2 CHCH 2 CH 2 OH. Sort by Relevance. It has a role as a polar solvent and a plant metabolite.
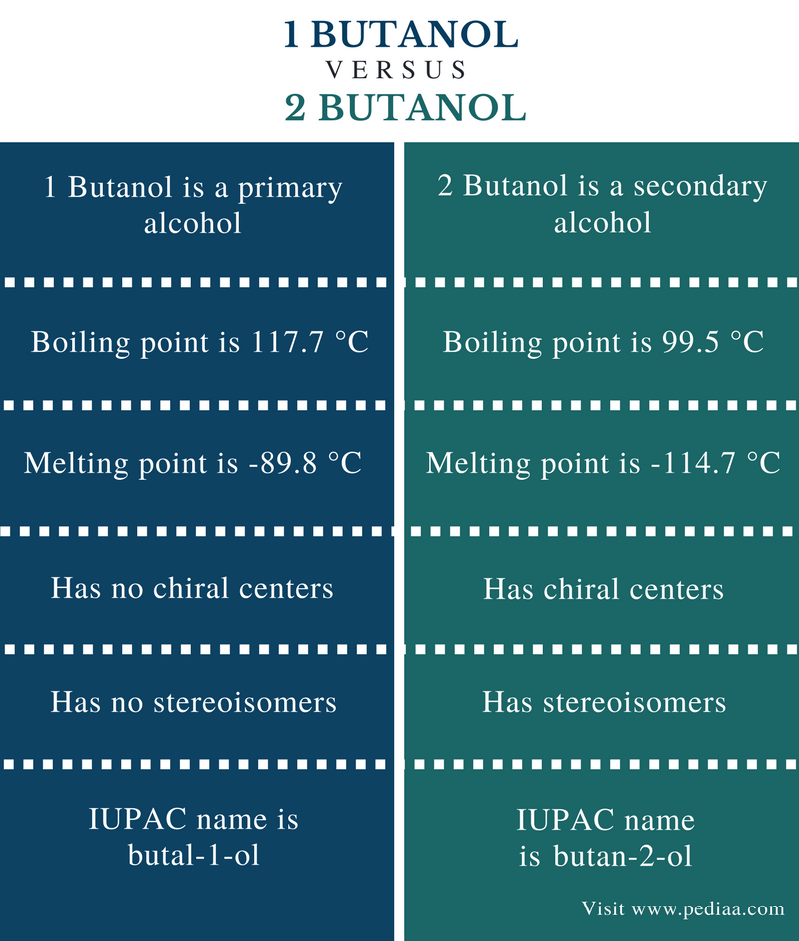 Source: pediaa.com
Source: pediaa.com
Hydrocarbons - Physical Data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. Sch4u Final Exam Review Organic Chemistry Answers. Hessel and Geiseler 1965. Hydrocarbons alcohols and acids - boiling points - Boiling temperature C and F with varying carbon number up to C33. 2-methyl-2-butanol 12-dimethylcyclohexanol 234-trimethylcyclopentanol 33-dimethyl-2-pentanol.

Chapter 3 Alcohols Phenols and Ethers 13 25 Reactions of Alcohols 26 Dehydration of Alcohols to Produce Alkenes Heating alcohols in concentrated sulfuric acid H2SO4 at 180C removes the OH group and a H from an adjacent carbon to produce an alkene with. It is one of several isomers of amyl alcohol pentanol. Known Compounds Boiling Point C Refractive Index n 20 D Methyl acetate 569 13614 Acetone 581 13591 Methanol 647 13292 2-propanol 784 13770 1-propanol 972 13840 2-butanol 98 13970 2-methyl-1-propanol 108 13960 1-butanol 118 13990 Some Thoughts on the Graphical Presentation of Data Graphical communication of data is one of those important skills that scientists must master. Kemme and Kreps 1969. In this experiment you will carry out several qualitative tests that will allow you to identify functional groups in organic molecules.

Personal Protective Equipment Eyes. Nicotine Salts Nicotine Salts Base PG Nicotine Salts Base. VG Nicotine Salts Base. The vapor pressure of a solution containing 13 grams of a nonvolatile solute in 100 grams of water at 25C is 27371 mmHg. The vapor pressure of water at 96C is 707 mmHg.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. Hydrocarbons - Physical Data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. Isoamyl alcohol is a colorless liquid with the formula C 5 H 12 O specifically H 3 C 2 CHCH 2 CH 2 OH. It is also known as isopentyl alcohol isopentanol or in the IUPAC recommended nomenclature 3-methyl-butan-1-olAn obsolete name for it was isobutyl carbinol. It has a role as a polar solvent and a plant metabolite.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. A covalent bonding b Non polar c low meltingboiling answer choices It is a soft 3-dimensional network solid that is a good conductor of electricity. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly. 5000 mm Hg Vapor Pressure. Kemme and Kreps 1969.
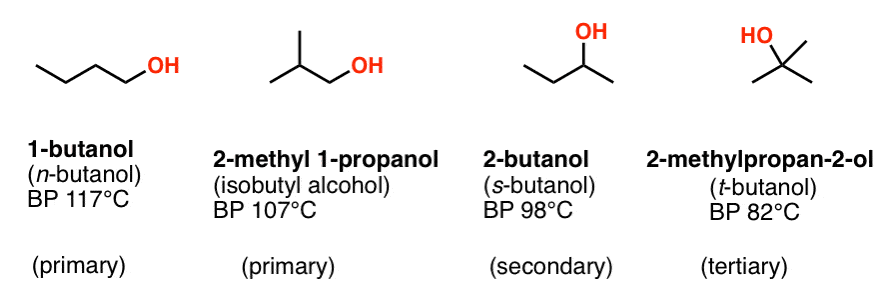 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Increasing boiling point and increasing solubility in water. 2-methyl-2-butanol 12-dimethylcyclohexanol 234-trimethylcyclopentanol 33-dimethyl-2-pentanol. It has a role as a polar solvent and a plant metabolite. It derives from a hydride of an isopentane. Determine the mass PbNO32 required to form a saturated solution in 200.
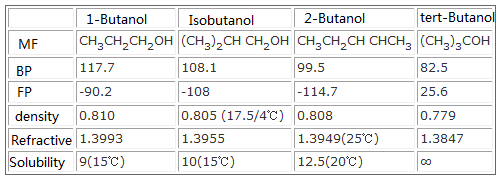 Source: chemicalbook.com
Source: chemicalbook.com
Increasing boiling point and increasing solubility in water. 3-methyl-2-butanol is a secondary alcohol that is 2-butanol carrying an additional methyl substituent at position 3. Hydrocarbons - Physical Data - Molweight melting and boiling point density flash point and autoignition temperature as well as number of carbon and hydrogen atoms in each molecule are given for 200 different hydrocarbons. Chapter 3 Alcohols Phenols and Ethers 13 25 Reactions of Alcohols 26 Dehydration of Alcohols to Produce Alkenes Heating alcohols in concentrated sulfuric acid H2SO4 at 180C removes the OH group and a H from an adjacent carbon to produce an alkene with. Tertiary Tertiary Secondary Secondary.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title 2 butanol boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.