2 bromopropane melting point
Home » datasheet » 2 bromopropane melting point2 bromopropane melting point
2 Bromopropane Melting Point. 2144 to 2171 F. Assume a 100 yield of product. Addition of a solution of HCl to a 02352-g sample of the compound of silver and carbon produced. C 14-dichlorobenzene para isomers are more symmetric and ortho and meta CBSE Class 12 Chemistry Solved Sample Paper 2021-22 Term-1 6.
 2 Bromopropane 99 75 26 3 From sigmaaldrich.com
2 Bromopropane 99 75 26 3 From sigmaaldrich.com
New Window-1734 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. Assume a 100 yield of product. Boiling point 48oC This molecule is non- polar. A 12-dichlorobenzene b 13 -dichlorobenzene c 14-dichlorobenzene d All isomers have the same melting points Ans 5. Enter the email address you signed up with and well email you a reset link. C 14-dichlorobenzene para isomers are more symmetric and ortho and meta CBSE Class 12 Chemistry Solved Sample Paper 2021-22 Term-1 6.
P-Dibromobenzenc has higher melting point than its o-isomer.
Std molar entropy S o 298 32702 J K 1 mol 1. Ch2noh name - e-mobility-testde. AFrenkel defect bSchottky defect cmetal deficiency defect dinterstitial defect. The freezing point depression constant Kfp of water is 186 ºCm. C 14-dichlorobenzene para isomers are more symmetric and ortho and meta CBSE Class 12 Chemistry Solved Sample Paper 2021-22 Term-1 6. Std molar entropy S o 298 32702 J K 1 mol 1.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Scandium has only one electron in the 3d. Std enthalpy of formation Δ f H 298. CH 3 CH 2 CH 2 Br propyl bromide 1-bromopropane. National Toxicology Program Chemical Repository Database. Ch2noh name - e-mobility-testde.

Std enthalpy of formation Δ f H 298. Which one of the following reactions is not explained by the. Which of the following isomer has the highest melting point. 2-Bromopropane also known as isopropyl bromide and 2-propyl bromide is the halogenated hydrocarbon with the formula CH 3 CHBrCH 3. 2-Bromopropane C3H7Br CID 6358 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
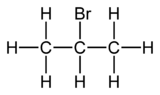 Source: en.wikipedia.org
Source: en.wikipedia.org
Aryl fluoride formation is not possible due to the high reactivity of the halogen fluorine. What mass of 2-bromopropane could be prepared from 255 g of propene. The hydrocarbons we have encountered so far have been composed of. Cl C C Cl H H Cl C C H H Cl The effect of EZ stereoisomerism on physical properties E-Z stereoisomers can have differing melting and. Since London forces get weaker with distance apart the greater the area of surface contact the stronger the London forces will be.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Cl C C Cl H H Cl C C H H Cl The effect of EZ stereoisomerism on physical properties E-Z stereoisomers can have differing melting and. It is due to symmetry of p-isomer which fits in crystal lattice better than the o-isomer. Assume the vant Hoff factor for Na2SO4 is 285. However it will react with moist silverI oxide and form water and a compound composed of silver and carbon. Assume a 100 yield of product.
 Source: fishersci.co.uk
Source: fishersci.co.uk
AMelting point of Phosphorous is less than that of Nitrogen bN2 is highly reactive while P4 is inert cNitrogen shows higher tendency of catenation than P dN-N is weaker than P-P 2. Manganese has weak metallic bonds due to electronic repulsion. Cl C C Cl H H Cl C C H H Cl The effect of EZ stereoisomerism on physical properties E-Z stereoisomers can have differing melting and. Compounds containing Cl F and C. 2-Bromopropane C3H7Br CID 6358 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.

AMelting point of Phosphorous is less than that of Nitrogen bN2 is highly reactive while P4 is inert cNitrogen shows higher tendency of catenation than P dN-N is weaker than P-P. The dipoles cancel out. Ch2noh name - e-mobility-testde. Why iodoform has appreciable antiseptic property. Name cycloalkanes given their formulas and write formulas for these compounds given their names.
 Source: chemsynthesis.com
Source: chemsynthesis.com
AMelting point of Phosphorous is less than that of Nitrogen bN2 is highly reactive while P4 is inert cNitrogen shows higher tendency of catenation than P dN-N is weaker than P-P 2. This is because of lack of hydrogen bonding. New Window-1734 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP. National Toxicology Program Chemical Repository Database. Therefore they can be easily separated from each other.
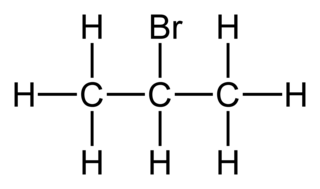 Source: wikiwand.com
Source: wikiwand.com
Which one of the following reactions is not explained by the. Which of the following isomer has the highest melting point. A 12 - dicholorbenzene b 13 - dichlorobenzene c 14 - dicholorbenzene d all isomers have. 2-Bromopropane is prepared by heating isopropanol with hydrobromic acid. Which of the following is a non-stoichiometric defect.
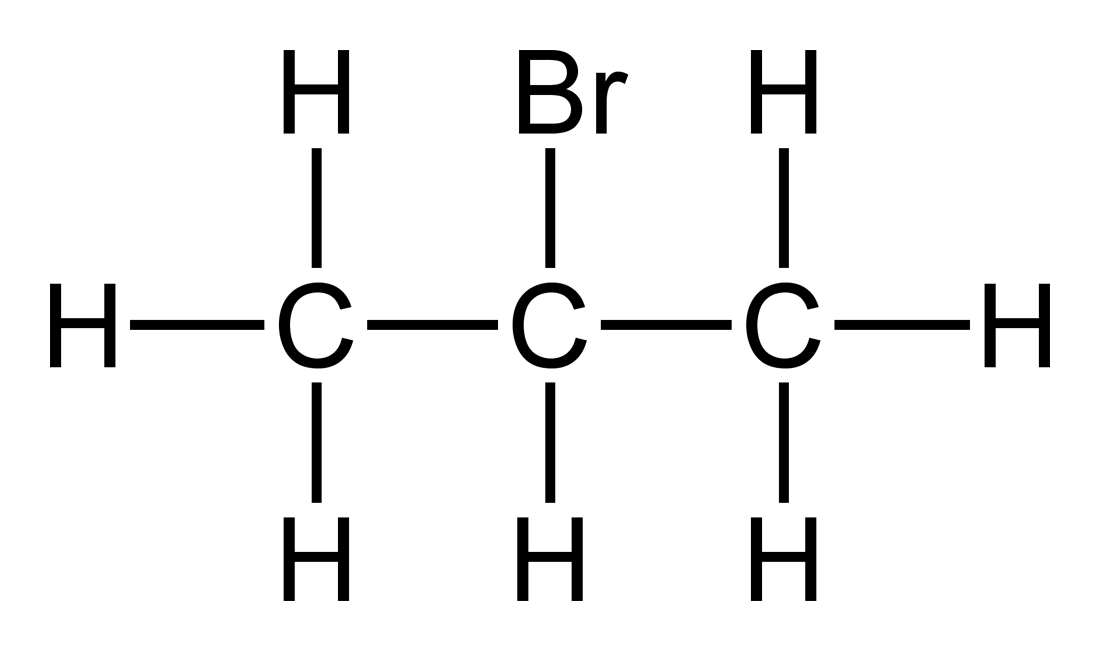 Source: en.wikipedia.org
Source: en.wikipedia.org
Preparation of aryl chloride and bromide is possible from this reaction. It is a colorless liquid. The dipoles cancel out. Compounds containing Cl F and C. A 12 - dicholorbenzene b 13 - dichlorobenzene c 14 - dicholorbenzene d all isomers have.
 Source: molinstincts.com
Source: molinstincts.com
Research Triangle Park North Carolina. The hydrocarbons we have encountered so far have been composed of. Addition of a solution of HCl to a 02352-g sample of the compound of silver and carbon produced. It is due to symmetry of p-isomer which fits in crystal lattice better than the o-isomer. It is used for introducing the isopropyl functional group in organic synthesis.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title 2 bromopropane melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.