10 hcl melting point
Home » datasheet » 10 hcl melting point10 hcl melting point
10 Hcl Melting Point. The melting point is specific for a given substance. Anhydrous 675 g100 mL 25 C 876 g100 mL 100 C hexahydrate 1238 g100 mL 25 C 1607 g100 mL 100 C Solubility. In this equation T FP is the freezing point depression the change in the freezing point that occurs when the solute dissolves in the solvent – and k f is the molal freezing point depression constant for the solvent. 105 1017 ww solution.
 Hydrochloric Acid Wikipedia From en.wikipedia.org
Hydrochloric Acid Wikipedia From en.wikipedia.org
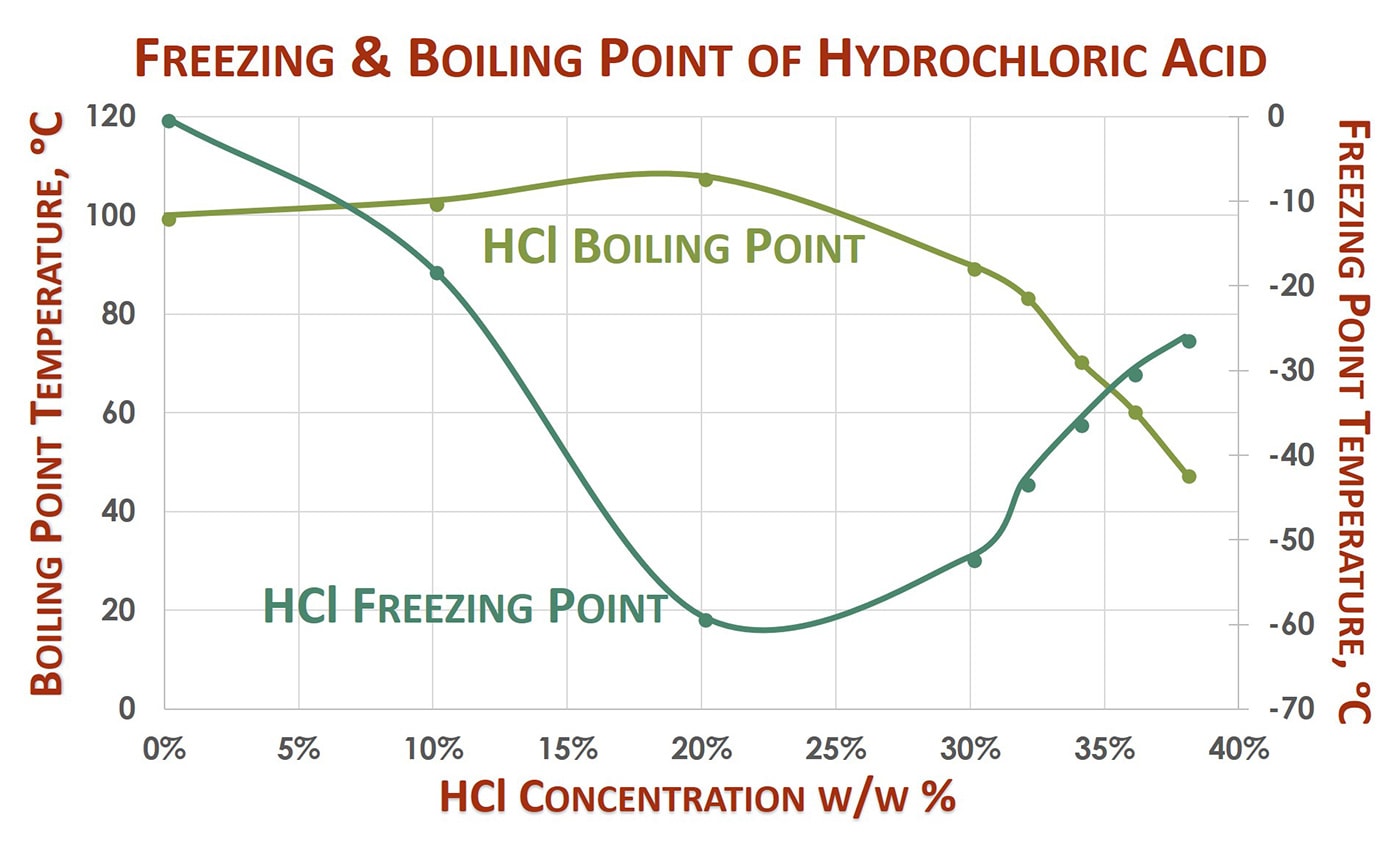
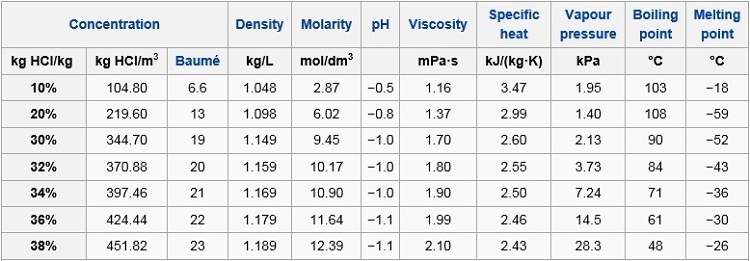
Record the volume of NaOH solution required to effect a colour change ie. T FP -k f m. I suppose the same factors are at play in the boiling point as well rigid structure leads to greater overall surface contact between molecules meaning stronger VDW. Substance Formula Melting point C Boiling temperature C Density 25C. Experimental Melting Point-1142 C OU Chemical Safety Data No. The physical properties such as density melting point PH boiling point depends on the molarity or concentration of HCl.
1001 C 1834 F.
For example the melting point of ice frozen water is 0 C. Branched more sphere-like - lower surface area lower boiling point. Predict the products of this reaction when it takes place in the presence of heat andor light. 110 01 N HCl. Equilibrium melting point of quartz is 1720 C with a flux-adjusted melting point of 1520 C. A solution of hydrogen chloride gas in water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
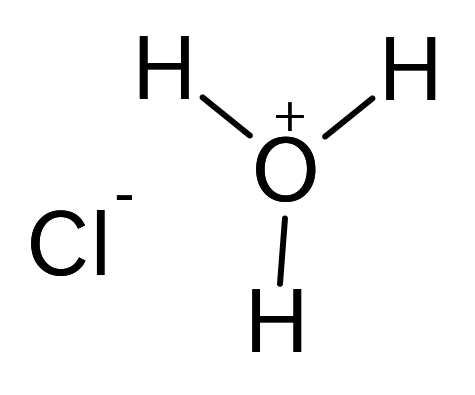
If this all seems rather ambiguous contradictory and imprecise well you have a point. T FP -k f m. I suppose the same factors are at play in the boiling point as well rigid structure leads to greater overall surface contact between molecules meaning stronger VDW. Molecular complexes of this kind commonly have a 5050 stoichiometry as shown but other integral ratios are known. Hydrochloric acid Structure HCl.
Source:
Highly branched vs. DCMs volatility and. In pure water near room temperature the concentration of H is about 10-7 molesliter which gives a pH of 7. B c from level 445 sample E301 405 cm. A from level 457 sample E313 413 cm depth.
 Source: protank.com
Source: protank.com
The higher normal boiling point of HCl 188 K compared to F 2 85 K is a reflection of the greater strength of dipole-dipole attractions between HCl molecules compared to the attractions between nonpolar F 2 molecules. All our masses were obtained accurately by carefully using the analytical balance. At this point θ is approximately. Halogenation reactions are a type of substitution reaction. The melting point is specific for a given substance.
 Source: www2.atmos.umd.edu
Source: www2.atmos.umd.edu
-1714 C 1081 solution. Platinum is an allotrope of carbon is the hardest natural substance known and has a very. I suppose the same factors are at play in the boiling point as well rigid structure leads to greater overall surface contact between molecules meaning stronger VDW. 1001 C 1834 F. Highly branched vs.
 Source: hydro-land.com
Source: hydro-land.com
We rinsed the burette set so as to clear any. By contrast the ionic solid NaCl has a melting point of 800C. Pyridoxine Hydrochloride is the hydrochloride salt form of pyridoxine a water-soluble vitamin B. Boiling Point-84990 C at 760 mmHg Vapour. In addition to the potential complications noted above the simple process of taking a melting point may also be influenced by changes in.
I hope this answers your question. May be colored yellow by traces of iron chlorine and organic matter. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. The melting point depends on the pressure. Preparation of Hydrochloric acid HCl.
Source:
See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. The AB complex has a melting point of 54 ºC and the phase diagram displays two eutectic points the first at 50 ºC the second at 30 ºC. Substance A is malleable ductile conducts electricity well and has a melting point of 1135 C. Cyclobenzaprine hydrochloride is a white crystalline tricyclic amine salt with the empirical formula C 20 H 21 NHCl and a molecular weight of 3119. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
Source: quora.com
967 C 1421 F. Chem 393 1967 396-397 NIST Spectra nist ri. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen. Chloroform and carbon tetrachloride as well as hydrogen chloride as a byproduct. B c from level 445 sample E301 405 cm.
 Source: sciencedirect.com
Source: sciencedirect.com
Acidity pK a 4 hexahydrate Magnetic susceptibility χ. I suppose the same factors are at play in the boiling point as well rigid structure leads to greater overall surface contact between molecules meaning stronger VDW. Melting point - the temperature at which a solid turns into a liquid. Chem 393 1967 396-397 NIST Spectra nist ri. Record the volume of NaOH solution required to effect a colour change ie.

967 C 1421 F. May be colored yellow by traces of iron chlorine and organic matter. This vitamin is essential to red blood cell nervous system and immune systems. Branched more sphere-like better stacking higher melting point. We rinsed the burette set so as to clear any.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title 10 hcl melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.