1 pentanol boiling point
Home » datasheet » 1 pentanol boiling point1 pentanol boiling point
1 Pentanol Boiling Point. The primary aim of the cards is to promote the safe use of chemicals in the workplace. Set up the distillation equipment as in the diagram on the right. It is used as a flavoring agent as a paint and lacquer solvent and in the preparation of penicillin. Solid-liquid and liquid-liquid extractions are commonly performed by batch and continuous processes.
 3 Trends That Affect Boiling Points Chemistry By Mukesh Sharma From chemistrybymukesh.blogspot.com
3 Trends That Affect Boiling Points Chemistry By Mukesh Sharma From chemistrybymukesh.blogspot.com
Rank the following compounds in order of decreasing boiling point. In this example COOH OH O O H 2O. It is an. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. The removal of caffeine from coffee beans with dichloromethane is an example of a solid liquid extraction. The procedure for the distillation of the mixture containing the ester is outlined below.
It is used as a flavoring agent as a paint and lacquer solvent and in the preparation of penicillin.
To the mixture in the round bottom flask as prepared above add boiling chips to prevent bumping. Chapter 6 Amines and Amides 23 Some Important Alkaloids 24 Important Alkaloids N N N N O CH3 O CH3 CH3 Caffeine Found in the seeds of Coffea arabica roasted coffee beans. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. I the mixture in the round bottom flask is best heated using a. To the mixture in the round bottom flask as prepared above add boiling chips to prevent bumping. 1-Pentanol l C 5 H 12 O-3516.
 Source: researchgate.net
Source: researchgate.net
20 During the manufacture and use of plastic materials and articles reaction and. Crystal violet may be removed from a water solution by liquid-liquid extraction with n-amyl alcohol 1-pentanol. Boiling points of alcohols. In this example COOH OH O O H 2O. Such a large difference in boiling points indicates that molecules of ethanol are attracted to one another much more strongly.

A sharp boiling point is an indication of the purity of the ester. It also shows that the boiling point of alcohols increase with the number of carbon atoms. 20 During the manufacture and use of plastic materials and articles reaction and. In this example COOH OH O O H 2O. 1-pentanol or 1-aminopentane.

It can also apply to hydrogen bonding molecules like alcohols compare the boiling points of 1-pentanol to 2-pentanol and 3-pentanol for instance. A liquid state that is more stable that is one that is held together by stronger dipole forces will have a higher boiling point since it takes more energy to break these intermolecular forces. Any potential health risk in the final material or article arising from their use should be assessed by the manufacturer in accordance with internationally recognised scientific principles on risk assessment. Chapter 6 Amines and Amides 23 Some Important Alkaloids 24 Important Alkaloids N N N N O CH3 O CH3 CH3 Caffeine Found in the seeds of Coffea arabica roasted coffee beans. The boiling points of alcohols are much higher than those of alkanes with similar molecular weights.

1-Pentanol g C 5 H 12 O-2946. Inhibits the action of phosphodiesterase an enzyme which inhibits cyclic adenosine. 1-pentanol or 1-aminopentane 23 Some Important Alkaloids 24 Important Alkaloids N N N N O CH3 O CH3 CH3 Caffeine Found in the seeds of Coffea arabica roasted coffee beans. 1-Pentanol g C 5 H 12 O-2946. 2-Pentanol g C 5 H 12 O-311.
 Source: chemistrybymukesh.blogspot.com
Source: chemistrybymukesh.blogspot.com
22 g100 mL. Get 247 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. It is the straight-chain form of amyl alcohol one of 8 isomers with that formula. The effect of van der Waals forces.

1-pentanol or 1-aminopentane. Set up the distillation equipment as in the diagram on the right. It can also apply to hydrogen bonding molecules like alcohols compare the boiling points of 1-pentanol to 2-pentanol and 3-pentanol for instance. It also shows that the boiling point of alcohols increase with the number of carbon atoms. 76000 mm Hg Acid Value.
 Source: fishersci.co.uk
Source: fishersci.co.uk
CH 3 CH 2 2 CONH 2. It is used as a flavoring agent as a paint and lacquer solvent and in the preparation of penicillin. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F. CH 3 CH 2 3 CHO. All of the compounds have about the same molecular weight propanoic acid diethylamine 1-butanol.

CH 3 CO 2 C 2 H 5. Further increase in volume has to be carried out since one sees the second plateau the point where the boiling point of second component takes place The ratio of purity of products is xy where x is the volume of liquid where first plateau. Hints for logging in with Active Directory. For example ethanol with a molecular weight MW of 46 has a boiling point of 78 C 173 F whereas propane MW 44 has a boiling point of 42 C 44 F. It is an.
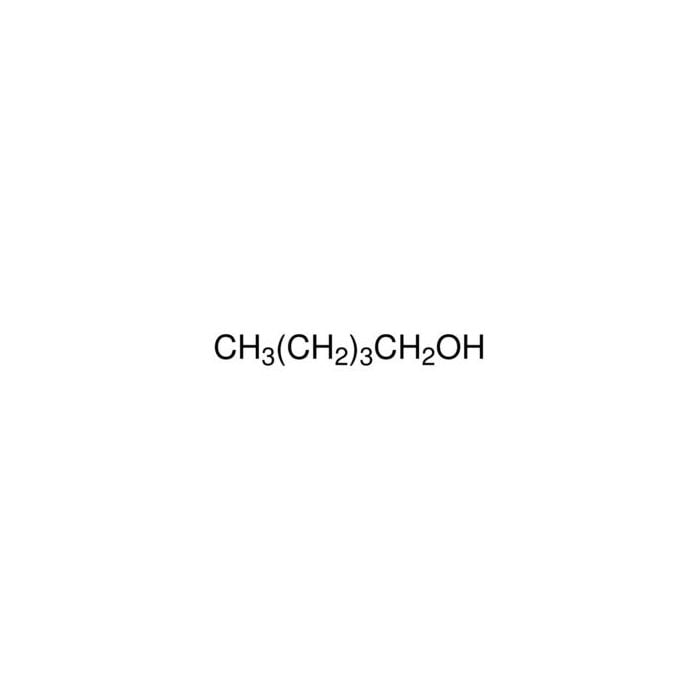 Source: lab.honeywell.com
Source: lab.honeywell.com
3-Pentanol g C 5 H 12 O-3149. Any potential health risk in the final material or article arising from their use should be assessed by the manufacturer in accordance with internationally recognised scientific principles on risk assessment. The main target users are workers and those responsible for occupational safety and health. This volume is designated as x. 20 During the manufacture and use of plastic materials and articles reaction and.
 Source: tcichemicals.com
Source: tcichemicals.com
It is the straight-chain form of amyl alcohol one of 8 isomers with that formula. It is an. CH 3 CH 2 2 CO 2 H. Even after increase in volume the boiling point remains constant and at a particular volume of liquid it starts to rise. Rank the following compounds in order of decreasing boiling point.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title 1 pentanol boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.