1 methylcyclohexene boiling point
Home » datasheet » 1 methylcyclohexene boiling point1 methylcyclohexene boiling point
1 Methylcyclohexene Boiling Point. Attack of the bromine radical on the more substituted carbon would result in a new free radical on a secondary carbon. Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene and is the major component in the oil of citrus fruit peels. Explain why propanol has higher boiling point than that of the hydrocarbon butane. 3 Carbocation D can undergo a type of reaction called a.

1 mark carbocation D carbocation D. Name of compo-und structure molwt. Limonene - is an oral dietary supplement containing a natural cyclic monoterpene and a major component of the oil extracted from citrus peels with potential chemopreventive and antineoplastic activitiesUpon oral administration D-limonene activates aldehyde dehydrogenase 3A1 ALDH3A1 thereby decreasing aldehyde level. To this solution A an equal volume of 01 molal aqueous barium chloride solution is added to make a new solution B. The decreasing order of boiling point of the following alcohols is a 3-methylbuan-2-ol 2-methylbutan-2-ol pentan-1-ol b Pentan-1-ol 3-methylbutan-2-ol 2-methylbutan-2-ol c 2-methylbutan-2-ol 3-methylbutan-2-ol pentan-1-ol d 2-methylbutan-2-ol pental-1-ol 3-methylbutan-2-ol. Limonene is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene and is the major component in the oil of citrus fruit peels.
Which of the following is true about alkanes.
The product is a mixture of 1-methylcyclohexene and 3-methyl. Most elimination reactions follow Zaitsevs rule. The addition follows Markownikovs rule. Ozone is a molecule that most people are familiar with hearing about either because it is missing. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. They are linked together exclusively by single bonds.
 Source: us.vwr.com
Source: us.vwr.com
GC analysis indicated that the major product was 1-methylcyclohexene and that the minor product was 3-methylcyclohexene. Additionally since only two peaks were depicted on the GC graph it is considered unlikely for this reaction when done with the above procedure to produce methylenecyclohexane as second minor product. 1 mark carbocation D carbocation D. The boiling point of 1-methycyclohexene is 110. If the compound has a low boiling point it probably evaporated during elution.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The question is which atom of the double bond does the free radical attack. The boiling point of water in a 01 molal silver nitrate solution solution A is x ºC. Assume no change in volume during boiling. They are linked together exclusively by single bonds. 1 mark carbocation D carbocation D.
 Source: chemsynthesis.com
Source: chemsynthesis.com
3 Carbocation D can undergo a type of reaction called a. 13 13 Turn over IBMJun1774042 Do not write outside the box 0 7. The decreasing order of boiling point of the following alcohols is a 3-methylbuan-2-ol 2-methylbutan-2-ol pentan-1-ol b Pentan-1-ol 3-methylbutan-2-ol 2-methylbutan-2-ol c 2-methylbutan-2-ol 3-methylbutan-2-ol pentan-1-ol d 2-methylbutan-2-ol pental-1-ol 3-methylbutan-2-ol. Cis-and trans-2-methyl- Cyclo- hexanol. The boiling point of water in a 01 molal silver nitrate solution solution A is x ºC.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
This may protect salivary stemprogenitor cells SSPCs from toxic. Cis-and trans-2-methyl- Cyclo- hexanol. Having just talked about the oxidation ladder it makes sense to start going into reagents for oxidation and reduction reactions. Name of compo-und structure molwt. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products.
 Source: en.wikipedia.org
Source: en.wikipedia.org
They are unsaturated hydrocarbons. The boiling point of 1-methycyclohexene is 110. They are unsaturated hydrocarbons. Densities of the solutions A and B are the same as that of water and the soluble salts. The question is which atom of the double bond does the free radical attack.

For example 2-pentene boiling point 36texto textC was spotted in lane 1 of Figure 145c. It derives from a hydride of a p-menthane. You should expect that the more substituted alkene will be formed if at all possibleLike in the elimination reaction below for instance we get 80 of the tetrasubstituted alkene Zaitsev more substituted because there are 4. The addition follows Markownikovs rule. 3 Carbocation D can undergo a type of reaction called a.
 Source: chemsynthesis.com
Source: chemsynthesis.com
The major and minor. Assume no change in volume during boiling. They are linked together exclusively by single bonds. Additionally since only two peaks were depicted on the GC graph it is considered unlikely for this reaction when done with the above procedure to produce methylenecyclohexane as second minor product. They are unsaturated hydrocarbons.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Additionally since only two peaks were depicted on the GC graph it is considered unlikely for this reaction when done with the above procedure to produce methylenecyclohexane as second minor product. This may protect salivary stemprogenitor cells SSPCs from toxic. They are linked together exclusively by single bonds. It has a role as a human metabolite. The addition of HCl to methylenecyclohexane or the addition of HCl to 1-methylcyclohexene.
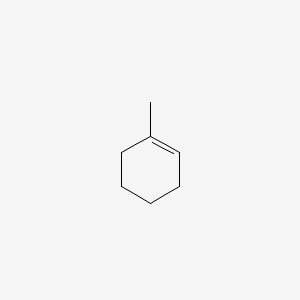
These are known are the 14-adducts because they add to the first and. You should expect that the more substituted alkene will be formed if at all possibleLike in the elimination reaction below for instance we get 80 of the tetrasubstituted alkene Zaitsev more substituted because there are 4. Question 8 predict the product for the following reaction. It did not stain with permanganate after elution even though the compound is reactive to the stain an undiluted uneluted sample of 2-pentene did stain somewhat on a scrap TLC plate Figure 245d. Draw the reaction of 1-methylcyclohexene treated with potassium permanganate in aqueous acid.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
125 mL of a solution of tribasic acid molecular weight 210 was. The bond could break two different ways after all. The decreasing order of boiling point of the following alcohols is a 3-methylbuan-2-ol 2-methylbutan-2-ol pentan-1-ol b Pentan-1-ol 3-methylbutan-2-ol 2-methylbutan-2-ol c 2-methylbutan-2-ol 3-methylbutan-2-ol pentan-1-ol d 2-methylbutan-2-ol pental-1-ol 3-methylbutan-2-ol. Which is more highly regioselective. 1-methylcyclohexene via carbocation D by drawing the structure of the missing intermediate all necessary curly arrows.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title 1 methylcyclohexene boiling point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.