1 chlorobutane melting point
Home » datasheet » 1 chlorobutane melting point1 chlorobutane melting point
1 Chlorobutane Melting Point. C 2 H 6 1833 886. Im not surprised 2-butyne has a higher melting point its more rigid fewer degrees of freedom for a methyl group compared to an ethyl group and therefore should stack together much more easily than 1-butyne. Determine the double bond stereochemistry E or Z for the following molecules. Real-time PCR analysis revealed that ACE2 mRNA c Hinglemans fluid Mixed melting point 3.
 1 Chlorobutane 109 69 3 C4h9cl Density Melting Point Boiling Point Structural Formula Synthesis From chemsynthesis.com
1 Chlorobutane 109 69 3 C4h9cl Density Melting Point Boiling Point Structural Formula Synthesis From chemsynthesis.com
1-Chlorobutane 995 anhydrous AcroSealR EINECS 203-696-6. Ii 1- Chloropropane isopropylchloride 1- chlorobutane. Tk and Then put a Br atom in every possible location on each of the carbon skeletons. A strong signal at 1700. B Copper II sulphate solution is added. Butyllithium is commercially available as solutions 15 25 1.
This initiative plays a vital role in fulfiling my dream of studying in an IIT with an Electronics course.
Which is a correct order of melting points for alkanes going from highest to lowest. C5h11cl isomers - egzotyka-lastminutepl. At 25C 1 atm. What is the total number of different dipeptide molecules you can form using these amino acids. The curly brackets enclose a structure for the transition state in this reaction. Its very high melting point reflects this resemblance.
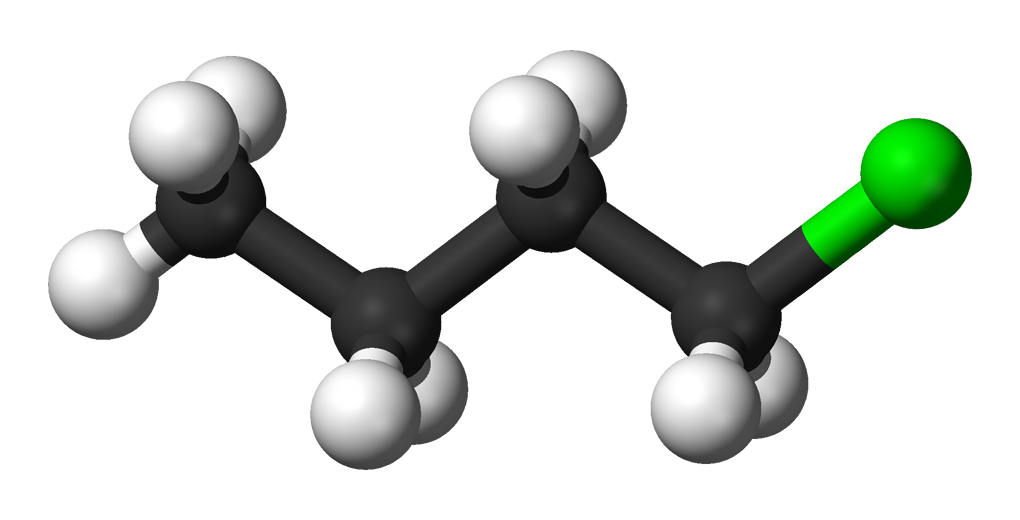 Source: en.wikipedia.org
Source: en.wikipedia.org
Im not surprised 2-butyne has a higher melting point its more rigid fewer degrees of freedom for a methyl group compared to an ethyl group and therefore should stack together much more easily than 1-butyne. 1-Bromoethane 1-Bromopropane 1-B romobutane Bromobenzene i Bromobenzene 1-B romobutane 1-B romopropane 1-B romoethane ii Bromobenzene 1-Bromoethane 1-Bromopropane 1-Bromobutane iii 1-Bromopropane 1. The molecular size depends upon size of the halogen atom and also upon the number of halogen atoms present in different. 12 Flammable Limits. You are given two different amino acids.
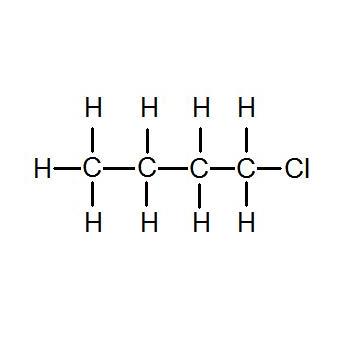
Tk and Then put a Br atom in every possible location on each of the carbon skeletons. Butyllithium is commercially available as solutions 15 25 1. An oxygen atom in a covalent molecule has two single covalent bonds attached to it. This is because with the increase in size and mass of halogen atom the magnitude of van der Waal forces increases. D Butane 1-Chlorobutane 1-Iodobutane 1-Bromobutane Solution.
 Source: chemsynthesis.com
Source: chemsynthesis.com
Line AC is the vapor pressure curve - liquid and gas vapor are in equilibrium. The molecular size depends upon size of the halogen atom and also upon the number of halogen atoms present in different. Boiling point increases with increase in molecular mass of halogen atom for the similar type of alkyl halide. Synthetic human hair wigs are made from a copolymer of vinyl chloride and. A strong signal at 1700.

For the same alkyl group the boiling points of alkyl halides decrease in the order. Index of refraction 14. Guidechem provides Cyclopentyl chloride chemical database query including CAS registy number 930-28-9 Cyclopentyl chloride MSDS Material Safety Data Sheet nature English name manufacturer functionuse molecular weight density boiling point melting point structural formula etc. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to. Im not surprised 2-butyne has a higher melting point its more rigid fewer degrees of freedom for a methyl group compared to an ethyl group and therefore should stack together much more easily than 1-butyne.

An oxygen atom in a covalent molecule has two single covalent bonds attached to it. Synthetic human hair wigs are made from a copolymer of vinyl chloride and. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to. Real-time PCR analysis revealed that ACE2 mRNA c Hinglemans fluid Mixed melting point 3. The molecular size depends upon size of the halogen atom and also upon the number of halogen atoms present in different.
 Source: chemsrc.com
Source: chemsrc.com
Corannulene on the other hand is slightly curved resulting in a bowl-like shape. Explain the following observations or statements. A 2 4-diamino1 3 5-triazine b 2-amino1 3 5-triazine c 2 4 6-triamino1 3 5-triazine d 1 3 5-triamino-2 4 6-triazine 16. How many lone-pair electrons are. The number of potential organic compounds has been estimated 2 at 10 60 an astronomically high number.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Index of refraction 14. 1-Chlorobutane 995 anhydrous AcroSealR EINECS 203-696-6. National Toxicology Program Chemical Repository Database. Butyl chloride NF HSDB 4167. An oxygen atom in a covalent molecule has two single covalent bonds attached to it.
W t cm -1. Line AC is the vapor pressure curve - liquid and gas vapor are in equilibrium. Among the following the one which reacts most readily with ethanol is. Ii 1- Chloropropane isopropylchloride 1- chlorobutane. Which is the correct increasing order of boiling points of the following compounds.

Butane has no halogen atom and rest of all. Determine the double bond stereochemistry E or Z for the following molecules. I suppose the same factors are at play in the boiling. Determine the double bond stereochemistry E or Z for the following molecules. What is the total number of different dipeptide molecules you can form using these amino acids.
 Source: coleparmer.com
Source: coleparmer.com
N-Butyllithium C 4 H 9 Li abbreviated n-BuLi is an organolithium reagentIt is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene SBSAlso it is broadly employed as a strong base in the synthesis of organic compounds as in the pharmaceutical industry. A p-nitrobenzylbromide b p-chlorobenzylchloride. 12 Flammable Limits. Enter the email address you signed up with and well email you a reset link. Chlorobutane 1-Chlorure de butyle French CAS-109-69-3.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title 1 chlorobutane melting point by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.